Carbon Accustomed with Carbon Nanotube and Zeolite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Α,Ω-Biphenylpolyynes)
1 ONE-POT SYNTHESIS AND CHARACTERIZATION OF POLYYNES END-CAPPED BY BIPHENYL GROUPS (α,ω-BIPHENYLPOLYYNES) Franco Cataldo1(*), Ornella Ursini2, Alberto Milani3 , Carlo S. Casari3 1Actinium Chemical Research Institute, Via Casilina 1626 A, Rome, Italy 2CNR - Istituto di Metodologie Chimiche, Montelibretti, Roma 3 Department of Energy, Politecnico di Milano via Ponzio 34/3, I-20133 Milano, Italy Abstract Stable polyyne chains terminated with biphenyl end groups (α,ω-biphenylpolyynes) were synthesized in a single step with an easy procedure by using the Cadiot-Chodkiewicz reaction conditions. The α,ω-biphenylpolyynes were separated through HPLC analysis and identified by means of their electronic absorption spectra. The α,ω-biphenylpolyynes were studied by FT-IR and Raman spectroscopy and the spectral interpretation was supported with DFT calculations. A peculiar stability of α,ω-biphenylpolyynes towards ozone was also observed. 1. Introduction The sensational report about the production of a long polyyne chain of 6000 carbon atoms enclosed in a double wall carbon nanotube (DWCNT) [1] has re-awakened the enthusiasm about carbyne, the fabulous carbon allotrope made by an infinitely long sequence of carbon atoms with sp hybridization (with either alternated single and triple bonds or cumulated double bonds). Earlier attempts to the synthesis of carbyne are reviewed for example in the book of Heimann at al. [2]. *Corresponding author. Tel: 0039-06-94368230. E-mail: [email protected] The chemical approach toward the synthesis of this long acetylenic structure for example through the Glaser coupling reaction invariably led to a crosslinked carbonaceous solid [3,4] whose solid state 13C-NMR analysis and Raman spectroscopy revealed indeed the presence of sp hybridized carbon 2 atoms but mixed with sp2 and sp3 hybridized carbon atoms due to undesired crosslinking reactions [5,6]. -

Nadia Merit List
NATIONAL MEANS‐CUM ‐MERIT SCHOLARSHIP EXAMINATION,2020 PAGE NO.1/56 GOVT. OF WEST BENGAL DIRECTORATE OF SCHOOL EDUCATION SCHOOL DISTRICT AND NAME WISE MERIT LIST OF SELECTED CANDIDATES CLASS‐VIII NAME OF ADDRESS OF ADDRESS OF QUOTA UDISE NAME OF SCHOOL DISABILITY MAT SAT SLNO ROLL NO. THE THE THE GENDER CASTE TOTAL DISTRICT CODE THE SCHOOL DISTRICT STATUS MARKS MARKS CANDIDATE CANDIDATE SCHOOL HOGALBERIA ADARSHA AYADANGA SHIKSHANIKETAN, ROAD,HOGALBARIA HOGALBERIA ADARSHA 1 123204713031 ABHIJIT SARKAR NADIA 19101007604 VILL+P.O- NADIA M SC NONE 49 23 72 ,HOGALBARIA , SHIKSHANIKETAN HOGOLBARIA DIST- NADIA 741122 NADIA W.B, PIN- 741122 KARIMPUR JAGANNATH HIGH BATHANPARA,KARI ABHIK KUMAR KARIMPUR JAGANNATH SCHOOL, VILL+P.O- 2 123204713013 MPUR,KARIMPUR , NADIA 19101001003 NADIA M GENERAL NONE 72 62 134 BISWAS HIGH SCHOOL KARIMPUR DIST- NADIA 741152 NADIA W.B, PIN- 741152 CHAKDAHA RAMLAL MAJDIA,MADANPUR, CHAKDAHA RAMLAL ACADEMY, P.O- 3 123204703069 ABHIRUP BISWAS CHAKDAHA , NADIA NADIA 19102500903 NADIA M GENERAL NONE 68 72 140 ACADEMY CHAKDAHA PIN- 741245 741222, PIN-741222 KRISHNAGANJ,KRIS KRISHNAGANJ A.S HNAGANJ,KRISHNA KRISHNAGANJ A.S HIGH HIGH SCHOOL, 4 123204705011 ABHISHEK BISWAS NADIA 19100601204 NADIA M SC NONE 59 54 113 GANJ , NADIA SCHOOL VILL=KRISHNAGANJ, 741506 PIN-741506 KAIKHALI HARITALA BAGULA PURBAPARA HANSKHALI HIGH SCHOOL, VILL- BAGULA PURBAPARA 5 123204709062 ABHRAJIT BOKSHI NADIA,HARITALA,HA NADIA 19101211705 BAGULA PURBAPARA NADIA M SC NONE 74 56 130 HIGH SCHOOL NSKHALI , NADIA P.O-BAGULA DIST - 741502 NADIA, PIN-741502 SUGAR MILL GOVT MODEL SCHOOL ROAD,PLASSEY GOVT MODEL SCHOOL NAKASHIPARA, PO 6 123204714024 ABU SOHEL SUGAR NADIA 19100322501 NADIA M GENERAL NONE 66 39 105 NAKASHIPARA BETHUADAHARI DIST MILL,KALIGANJ , NADIA, PIN-741126 NADIA 741157 CHAKDAHA RAMLAL SIMURALI,CHANDUR CHAKDAHA RAMLAL ACADEMY, P.O- 7 123204702057 ADIPTA MANDAL IA,CHAKDAHA , NADIA 19102500903 NADIA M SC NONE 67 46 113 ACADEMY CHAKDAHA PIN- NADIA 741248 741222, PIN-741222 NATIONAL MEANS‐CUM ‐MERIT SCHOLARSHIP EXAMINATION,2020 PAGE NO.2/56 GOVT. -

Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis Of
The Astrophysical Journal, 889:3 (26pp), 2020 January 20 https://doi.org/10.3847/1538-4357/ab616c © 2020. The American Astronomical Society. All rights reserved. Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis of Acetylene (C2H2) and D2-acetylene (C2D2) Ices Exposed to Ionizing Radiation via Ultraviolet–Visible Spectroscopy, Infrared Spectroscopy, and Reflectron Time-of-flight Mass Spectrometry Matthew J. Abplanalp1,2 and Ralf I. Kaiser1,2 1 W. M. Keck Research Laboratory in Astrochemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA 2 Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA Received 2019 October 4; revised 2019 December 7; accepted 2019 December 10; published 2020 January 20 Abstract The processing of the simple hydrocarbon ice, acetylene (C2H2/C2D2), via energetic electrons, thus simulating the processes in the track of galactic cosmic-ray particles penetrating solid matter, was carried out in an ultrahigh vacuum surface apparatus. The chemical evolution of the ices was monitored online and in situ utilizing Fourier transform infrared spectroscopy (FTIR) and ultraviolet–visible spectroscopy and, during temperature programmed desorption, via a quadrupole mass spectrometer with an electron impact ionization source (EI-QMS) and a reflectron time-of-flight mass spectrometer utilizing single-photon photoionization (SPI-ReTOF-MS) along with resonance-enhanced multiphoton photoionization (REMPI-ReTOF-MS). The confirmation of previous in situ studies of ethylene ice irradiation -

A Post-Buckminsterfullerene View of Carbon Chemistry
A POST-BUCKMINSTERFULLERENE VIEW OF CARBON CHEMISTRY Harold Kroto School of Chemistry and Molecular Sciences, University of Sussex, Brighton, BNI 9QJ UK Keywords: Cs0, Fullerenes, carbon particles INTRODUCTION The discovery of c60 Buckminsterfullerene, Fig 1, has its origins in a research programme involving synthetic chemistry, microwave spectroscopy and radioastronomyl. In 1915, at Sussex (with David Walton), the long chain polyyne H-CeC-CsC-CsN was synthesised and studied by microwave spectroscopy. Subsequently, with Takeshi Oka and NRC(0ttawa) astronomers, the molecule was discovered in space, Fig 2, by radioastronomy using the laboratory microwave frequencies. This discovery led on to the detection of the even longer carbon chain molecules HCTN, HCgN and HCl.lN in the space between the stars2. Further work aimed at understanding the formation of the chains in space focussed attention on the possibility that they are produced at the same time as carbon dust in red giant stars1,*. During experiments at Rice University in 1985 (with James Heath, Sean O'Brien, Robert Curl and Richard Smalley), designed to simulate the conditions in these stars and explore their capacity for carbon chain formation, the exciting discovery that C60 was remarkably stable was made3. It was found that under conditions where almost all the atoms in a carbon plasma had nucleated to form microparticles the molecule c60 remained behind - together with some CTO. This result was, as is now well 'known, rationalised on the basis of the closed cage structure shown in Fig 1. It was proposed that the geodesic and aromatic factors inherent in such a structure could account for the stability of the molecule. -

Pdf 372.94 Kb
The Development of Molecular Wires PART 11: ROLE OF RUTHENIUM AND OSMIUM POLYPYRIDINE COMPLEXES FOR FAST VECTORIAL ELECTRON TRANSFER By V. Grosshenny, A. Harriman, M. Hissler and R. Ziessel Ecole Europkenne de Hautes Etudes des Industries Chimique de Strasbourg, Universitk Louis Pasteur, Laboratoire de Chimie d’Electronique et de Photonique Molkculaires, Strasbourg, France The concludingpart of thispaper on the use of ruthenium(ZI) and osmium(II) polypyridyl complexes, as molecular sized terminal subunits that are linked together bypolyyne bridges functioning as molecular girders to retain the stereo- chemical rigidity, deals with the process of electron transfer between the subunits and considers the benefits conferred by the use of polyyne bridges. The ruthe- nium and osmium complexes have properties which aid the selective promo- tion of an electron from the metal to the bridging ligand, together with amenable absorption and emission spectral pro+, and facile oxidation-reduction processes. This makes them promising candidates for vectorial electron transfer. Future work to extend the lengths of the linkages, to ensure unidirectional and long- range electron tunnelling, and to anchor the wires to supports is discussed. These are the necessary requirements for the development of molecular wiring made from these materials forfuture use with molecular-scale electronic devices. The first part of this paper introduced the sub- metal centres, must occur between more widely- ject of molecular wires and considered the struc- spaced reactants and it displays a more signifi- ture and chemistry of the complexes that can cant attenuation factor. be used for them, and other materials currently Interestingly, the insertion of a platinum(I1) believed to be the best for this purpose (13). -
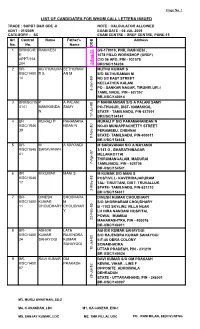
List of Candidates for Whom Call Letters Issued
Page No. 1 LIST OF CANDIDATES FOR WHOM CALL LETTERS ISSUED TRADE : SUPDT B&R GDE -II NOTE : CALCULATOR ALLOWED ADVT - 01/2009 EXAM DATE - 06 JUL 2009 CATEGORY - SC EXAM CENTRE - GREF CENTRE, PUNE-15 Srl Control Name Father's Address No. No. Name DOB 1 BRII/SC/R RAM KESI GS-179019, PNR, RAM KESI , E- 1078 FIELD WORKSHOP (GREF) APPT/154 C/O 56 APO, PIN - 931078 204 3-Aug-71 BRII/SC/154204 2 BR- MUTHUKUMA SETHURAM MUTHU KUMAR S II/SC/1400 R S AN M S/O SETHURAMAN M 14 NO 5/2 EAST STREET KEELATHEN KALAM PO - SANKAR NAGAR, TIRUNELUELI 8-Jan-80 TAMIL NADU, PIN - 627357 BR-II/SC/140014 3 BRII/SC/15 P A PALANI P MANIKANDAN S/O A PALANI SAMY 4141 MANIKANDA SAMY PO-THUSUR, DIST- NAMAKKAL N STATE - TAMILNADU, PIN-637001 17-Jul-88 BRII/SC/154141 4 BR MURALI P PARAMANA MURALI P S/O PARAMANANDAN N II/SC/1546 NDAN N NO-83 MUNIAPPACHETTY STREET 38 PERAMBEU, CHENNAI STATE- TAMILNADU, PIN-600011 9-Nov-80 BR II/SC/154638 5 BR- M A MAYANDI M SARAVANAN S/O A MAYANDI II/SC/1545 SARAVANAN 3/143 G , BHARATHINAGAR 41 MELAKKOTTAI THIRUMANGALAM, MADURAI 7-Apr-87 TAMILNADU, PIN - 625706 BR-II/SC/154541 6 BR M KUMAR MANI S M KUMAR S/O MANI S II/SC/1546 POST/VILL- KAVERIRAJAPURAM 17 TAL- TIRUTTANI, DIST- TRUVALLUR STATE- TAMILNADU, PIN-631210 2-May-83 BR II/SC/154617 7 BR- DINESH SHOBHARA DINESH KUMAR CHOUDHARY II/SC/1400 KUMAR M S/O SHOBHARAM CHOUDHARY 11 CHOUDHARY CHOUDHAR B -1102 SKYLINE VILLA NEAR Y LH HIRA NANDANI HOSPITAL POWAI, MUMBAI 22-Feb-85 MAHARASHTRA, PIN - 400076 BR-II/SC/140011 8 BR- ASHOK LATE ASHOK KUMAR SAHAYOGI II/SC/1400 KUMAR RAJENDRA S/O RAJENDRA KUMAR SAHAYOGI 24 SAHAYOGI KUMAR 5-F-46 OBRA COLONY SAHAYOGI SONABHADRA 10-Jul-82 UTTAR PRADESH, PIN - 231219 BR-II/SC/140024 9 BR- RAVI KUMAR OM RAVI KUMAR S/O OM PRAKASH II/SC/1400 PRAKASH KEWAL VIHAR , LINE F 07 OPPOSITE ADHOIWALA DEHRADUN 28-Jul-82 STATE - UTTARAKHAND, PIN - 248001 BR-II/SC/140007 M3. -

1 Manik Das S/O-Naresh Das, Vill-Tantla Roy Para, PO-Tatla, PS
Application for New Contract Carriage Permits of Auto-Rickshaw Meeting to be held on 14.01.2020 Memo No. 38(668)/MV Dt. 09.01.2020 Sl. Name & Address of the applicant No. 1 Manik Das S/O-Naresh Das, Vill-Tantla Roy para, P.O-Tatla, P.S-Chakdaha, Dist.-Nadia, Pin-741222 2 Manojit Das S/O-Manik Das, Vill-Tantla Raypara, P.O-Tantla, P.S-Chakdaha, Dist.-Nadia, Pin-741222 3 Soma Chanda S/O-Subhra Chanda, Vill-Dayalnagar, P.O-Nasra, Ranaghat, Dist.-Nadia, Pin-741202 4 Hossain Mandal S/O-Zamsher Mandal, Vill-Paschin Gopinathpur, P.S-Tehatta, Dist.-Nadia, Pin-741155 5 Sahidul Shekh S/O-Saheb Ali Shekh, Vill-Shyamnagar, P.O-Berij, P.S-Kotwali, Dist.-Nadia, Pin-741103 Mahadeb Ball S/O-Bijay Krishna Ball, Vill-Natungram, P.O-Nrisinghapur, P.S-Santipur, Dist.-Nadia, Pin- 6 741404 7 Aparna Chakraborty S/O-Debasish Chakraborty, Vill+ P.O+ P.S-Taherpur, Dist.-Nadia, Pin-741159 8 Tapan Biswas S/O-Madhusudan Biswas, Vill+ P.O-Ulashi, P.S-Hanskhali, Dist.-Nadia, Pin-741502 Sukanta Biswas S/O-Paritosh Biswas, Vill-Pakhiura, P.O-Kumari Ramnagar, P.S-Hanskhali, Dist.-Nadia, 9 Pin-741502 Puspa Mondal S/O-Sufal Mondal, Vill-Ulashi Paschimpara, P.O-Ulashi, P.S-Hanskhali, Dist.-Nadia, Pin- 10 741502 11 Dilip Biswas S/O-Madhusudan Biswas, Vill+ P.O-Ulashi, P.S-Hanskhali, Dist.-Nadia, Pin-741502 12 Pintu Saha S/O-Nimai Ch.Saha, Vill-Gournagar, P.O-Bagula, P.S-Hanskhali, Dist.-Nadia, Pin-741502 13 Sujit Biswas S/O-Subhas Biswas, Vill-Khanpara, P.O-Birnagar, P.S-Taherpur, Dist.-Nadia, Pin-741127 Bhakta Das S/O-Bimal Das, Vill-Chandpur Paschimpara, P.O-chandanpur, -

Chemistry and Applications of Metal-Organic Materials A
CHEMISTRY AND APPLICATIONS OF METAL-ORGANIC MATERIALS A Dissertation by DAN ZHAO Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY December 2010 Major Subject: Chemistry Chemistry and Applications of Metal-Organic Materials Copyright 2010 Dan Zhao CHEMISTRY AND APPLICATIONS OF METAL-ORGANIC MATERIALS A Dissertation by DAN ZHAO Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Approved by: Chair of Committee, Hong-Cai Zhou Committee Members, Abraham Clearfield François P. Gabbaï Hung-Jue Sue Head of Department, David H. Russell December 2010 Major Subject: Chemistry iii ABSTRACT Chemistry and Applications of Metal-Organic Materials. (December 2010) Dan Zhao, B.A., Zhejiang University; M.S., Zhejiang University Chair of Advisory Committee: Dr. Hong-Cai Zhou Developing the synthetic control required for the intentional 3-D arrangement of atoms remains a holy grail in crystal engineering and materials chemistry. The explosive development of metal-organic materials in recent decades has shed light on the above problem. Their properties can be tuned by varying the organic and/or inorganic building units. In addition, their crystallinity makes it possible to determine their structures via the X-ray diffraction method. This dissertation will focus on the chemistry and applications of two kinds of metal-organic materials, namely, metal-organic frameworks (MOFs) and metal-organic polyhedra (MOP). MOFs are coordination polymers. Their permanent porosity makes them a good “gas sponge”. In the first section, an isoreticular series of MOFs with dendritic hexa- carboxylate ligands has been synthesized and characterized structurally. -

ORDER SHEET Nature of the Case :- Arbitration Case
West Bengal Form No. 270 ORDER SHEET (RULE 129 OF THE RECORDS MANUAL, 1971) Order Sheet, Dated from 22/12/2015 to District:- Nadia. Arbitration Case No.XXXV/Arbi/2015 arising out of L. A. Case No.87/LA/NHAI/Hari/10-11 Nature of the case :- Arbitration case Serial number and Order and signature of Officer. Note of action date of Order taken on Order. 1 Whereas, it appears that 16 [Sixteen] awardees of 22.12.2015 Simhat Mouza, J. L. No.15 under P.S.- Haringhata Dist.- Nadia have submitted petitions under the provisior.s of the National Highways Act, 1956 [48 of 1956] and The Arbitration and Conciliation Act, 1996 [26 of 1996] with a prayer for reconsideration of market value of land as acquired u/s 3D of the National Highways Act, 1956 and for re-determination of amount payable Dictated, Corrected as compensation in connection with and signed by me Aush/Aman, & Bari/Viti, class of land u/s 3G of the said Act. Now, I, Sri. Vijay Bharti, IAS, the District Magistrate, Nadia, being appointed as Arbitrator by the National Highways I Authority of India u/s 3(G)5 of the N.H / ct.- 1956 do, hereby, request the A.D.M [L.A], Nadia & Competent Authority under the said Act to arrange for issuance of notices to the petitioners District Magistrate, for appearing on 29.12.2015 at 1:00 P.M. at the Nadia Zilla Parishad Nadia, Conference Hall, Krishnagar, Nadia before me to participate in the arbitration hearing. & (94 L. -

Containment Zones of Nadia
Nadia District Sl. No. Police District Sub-Div. Block G.P. Broad Based Containment Zone (a) (b) (c) (d) (e) (f) MAYAPUR BAMUNPUKUR-I GP of the area pertaining to Polling station No (s). & MAYAPUR Name (s): 14, Mayapur Thakur Bhakti Vinod Nimna Buniyadi Primary School, ROOM 1 Krishnagar Krishnagar Sadar NABADWIP BAMUNPUKUR-I NO. 1 including remaining polling areas of that polling station of 84, Nabadwip Assembly Constituency Banpur Fulbari of the area pertaining to Polling station No (s). & Name (s): 34 Fulbari 2 Krishnagar Krishnagar Sadar Krishnaganj MatiaryBanpur Pry School Room No-2 including remaining polling areas of that polling station of 88, Krishnaganj Assembly Constituency. Itkholapara of the area pertaining to Polling station No (s). & Name (s): 180 - Dr. B.R 3 Krishnagar Tehatta Tehatta-I BETAI-I Ambedkar College including remaining polling areas of that polling station of 78, Tehatta Assembly Constituency. A-11/296 of the area pertaining to Polling station No (s). & Name (s): 179, Kalyani 4 Ranaghat Kalyani Kalyani Municipality Ward no.-16 Sikshayatan School including remaining polling areas of that polling station of 92, Kalyani Assembly Constituency. Ward no.-13 of the area pertaining to Polling station No (s). & Name (s): 182, Swastha 5 Ranaghat Kalyani Kalyani Municipality Ward no.-13 O Paribar Kalyan Prashikhan Kendra (Room No-2) including remaining polling areas of that polling station of 92, Kalyani Assembly Constituency. B-2/369 of the area pertaining to Polling station No (s). & Name (s): 205 (Bidhan 6 Ranaghat Kalyani Kalyani Municipality Ward no.-08 Chandra Memorial Girls High School,Room no.-1) including remaining polling areas of that polling station of 92, Kalyani Assembly Constituency. -

Computational Chemistry
518 CHIMIA 2012, 66, No. 7/8 Computational Chemistry doi:10.2533/chimia.2012.518 Chimia 66 (2012) 518–531 © Schweizerische Chemische Gesellschaft ComputationalChemistry ComputationalChemistry Computational Chemistry,Talk 155 Computational Chemistry,Talk 156 ExploringPhotoinduced Intermolecular Electrontransferwith Local Control Theory in Trajectory-based Nonadiabatic Dynamics Molecular Dynamics Simulations B. F. E. Curchod1,T.J.Penfold1,2,3,U.Rothlisberger1,and I. Tavernelli1 A. Gamiz-Hernandez1,E.Vauthey2,M.Meuwly1 1Laboratory of Computational Chemistry and Biochemistry, Ecole Poly- technique Fédérale de Lausanne, CH-1015 Lausanne, Switzerland 1UniversityofBasel/DepartmentofChemistry, Klingelbergstr. 80, CH-4055 2Laboratoire de Spectroscopie Ultrarapide, Ecole Polytechnique Fédérale de Basel, Switzerland Lausanne, CH-1015 Lausanne, Switzerland 2UniversityofGeneva/DepartmentofPhysicalChemistry, 30 quaiErnest- 3SwissFEL, Paul Scherrer Inst., CH-5232 Villigen, Switzerland. Ansermet, CH-1211 Geneva 4, Switzerland In the last years, linear-response time-dependent density functional theory (LR-TDDFT) has become widely used for the calculation of vertical elec- Understandingthe dynamics of photoinduced chemical processes involving tronic excitation energies of medium to large size molecular systems in gas electron transfer(ET)isacrucialstep for the designofnew technologies and condensed phases. In addition, excited state LR-TDDFT forces and such as efficient solar energy conversion devices. Photoinduced ET pro- nonadiabatic couplings have also -

Exciton Radiative Lifetime in a Monoatomic Carbon Chain
Exciton radiative lifetime in a monoatomic carbon chain Stella Kutrovskaya,1,2,3 Sevak Demirchyan,1,2 Anton Osipov,3,4 Stepan Baryshev, 5 Anton Zasedatelev,5,6 Pavlos Lagoudakis,5,6 and Alexey Kavokin1,2,6,7,8 1 School of Science, Westlake University, 18 Shilongshan Road, Hangzhou 310024, Zhejiang Province, China 2 Institute of Natural Sciences, Westlake Institute for Advanced Study, 18 Shilongshan Road, Hangzhou 310024, Zhejiang Province, China 3 Department of Physics and Applied Mathematics, Stoletov Vladimir State University, 600000 Gorkii street, Vladimir, Russia 4 ILIT RAS | Branch of FSRC \Crystallography and Photonics" RAS,1 Svyatoozerskaya, Shatura, 140700, Moscow region, Russia 5 Skolkovo Institute of Science and Technology, 30 Bolshoy Boulevard, bld. 1, 121205 Moscow, Russia 6 Physics and Astronomy, University of Southampton, Highfield, Southampton, SO171BJ, United Kingdom 7 Russian Quantum Center, Skolkovo IC, Bolshoy Bulvar 30, bld. 1, Moscow 121205, Russia 8 NTI Center for Quantum Communications, National University of Science and Technology MISiS, Moscow 119049, Russia Linear carbon-based materials such as polyyne and cumulene oligomers provide a versatile platform for nano- physics and engineering. Direct gap quasi-1D polyyne structures are promising for the observation of strong and unusual excitonic effects arising due to the two-dimensional quantum confinement. Recently, we reported on the observation of sharp exciton peaks in low temperature photoluminescence spectra of polyyne chains [1]. Here, we analyse the time-resolved optical response of this system. We extend the non-local dielectric response theory to predict the exciton radiative lifetime dependence on the bandgap value and on the length of the chain.