Ice-Core Records and Ozone Depletion—Potential for a Proxy Ozone Record Paul Andrew Mayewski University of Maine, [email protected]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multi-Scale, Multi-Proxy Investigation of Late Holocene Tropical Cyclone Activity in the Western North Atlantic Basin
Multi-Scale, Multi-Proxy Investigation of Late Holocene Tropical Cyclone Activity in the Western North Atlantic Basin François Oliva Thesis submitted to the Faculty of Graduate and Postdoctoral Studies in partial fulfillment of the requirements for the Doctorate of Philosophy in Geography Department of Geography, Environment and Geomatics Faculty of Arts University of Ottawa Supervisors: Dr. André E. Viau Dr. Matthew C. Peros Thesis Committee: Dr. Luke Copland Dr. Denis Lacelle Dr. Michael Sawada Dr. Francine McCarthy © François Oliva, Ottawa, Canada, 2017 Abstract Paleotempestology, the study of past tropical cyclones (TCs) using geological proxy techniques, is a growing discipline that utilizes data from a broad range of sources. Most paleotempestological studies have been conducted using “established proxies”, such as grain-size analysis, loss-on-ignition, and micropaleontological indicators. More recently researchers have been applying more advanced geochemical analyses, such as X-ray fluorescence (XRF) core scanning and stable isotopic geochemistry to generate new paleotempestological records. This is presented as a four article-type thesis that investigates how changing climate conditions have impacted the frequency and paths of tropical cyclones in the western North Atlantic basin on different spatial and temporal scales. The first article (Chapter 2; Oliva et al., 2017, Prog Phys Geog) provides an in-depth and up-to- date literature review of the current state of paleotempestological studies in the western North Atlantic basin. The assumptions, strengths and limitations of paleotempestological studies are discussed. Moreover, this article discusses innovative venues for paleotempestological research that will lead to a better understanding of TC dynamics under future climate change scenarios. -

Lecture 21: Glaciers and Paleoclimate Read: Chapter 15 Homework Due Thursday Nov
Learning Objectives (LO) Lecture 21: Glaciers and Paleoclimate Read: Chapter 15 Homework due Thursday Nov. 12 What we’ll learn today:! 1. 1. Glaciers and where they occur! 2. 2. Compare depositional and erosional features of glaciers! 3. 3. Earth-Sun orbital parameters, relevance to interglacial periods ! A glacier is a river of ice. Glaciers can range in size from: 100s of m (mountain glaciers) to 100s of km (continental ice sheets) Most glaciers are 1000s to 100,000s of years old! The Snowline is the lowest elevation of a perennial (2 yrs) snow field. Glaciers can only form above the snowline, where snow does not completely melt in the summer. Requirements: Cold temperatures Polar latitudes or high elevations Sufficient snow Flat area for snow to accumulate Permafrost is permanently frozen soil beneath a seasonal active layer that supports plant life Glaciers are made of compressed, recrystallized snow. Snow buildup in the zone of accumulation flows downhill into the zone of wastage. Glacier-Covered Areas Glacier Coverage (km2) No glaciers in Australia! 160,000 glaciers total 47 countries have glaciers 94% of Earth’s ice is in Greenland and Antarctica Mountain Glaciers are Retreating Worldwide The Antarctic Ice Sheet The Greenland Ice Sheet Glaciers flow downhill through ductile (plastic) deformation & by basal sliding. Brittle deformation near the surface makes cracks, or crevasses. Antarctic ice sheet: ductile flow extends into the ocean to form an ice shelf. Wilkins Ice shelf Breakup http://www.youtube.com/watch?v=XUltAHerfpk The Greenland Ice Sheet has fewer and smaller ice shelves. Erosional Features Unique erosional landforms remain after glaciers melt. -

Concentrations, Particle-Size Distributions, and Dry Deposition fluxes of Aerosol Trace Elements Over the Antarctic Peninsula in Austral Summer
Atmos. Chem. Phys., 21, 2105–2124, 2021 https://doi.org/10.5194/acp-21-2105-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Concentrations, particle-size distributions, and dry deposition fluxes of aerosol trace elements over the Antarctic Peninsula in austral summer Songyun Fan1, Yuan Gao1, Robert M. Sherrell2, Shun Yu1, and Kaixuan Bu2 1Department of Earth and Environmental Sciences, Rutgers University, Newark, NJ 07102, USA 2Department of Marine and Coastal Sciences, Rutgers University, New Brunswick, NJ 08901, USA Correspondence: Yuan Gao ([email protected]) Received: 1 July 2020 – Discussion started: 26 August 2020 Revised: 3 December 2020 – Accepted: 8 December 2020 – Published: 12 February 2021 Abstract. Size-segregated particulate air samples were col- sition processes may play a minor role in determining trace lected during the austral summer of 2016–2017 at Palmer element concentrations in surface seawater over the conti- Station on Anvers Island, western Antarctic Peninsula, to nental shelf of the western Antarctic Peninsula. characterize trace elements in aerosols. Trace elements in aerosol samples – including Al, P, Ca, Ti, V, Mn, Ni, Cu, Zn, Ce, and Pb – were determined by total digestion and a 1 Introduction sector field inductively coupled plasma mass spectrometer (SF-ICP-MS). The crustal enrichment factors (EFcrust) and Aerosols affect the climate through direct and indirect ra- k-means clustering results of particle-size distributions show diative forcing (Kaufman et al., 2002). The extent of such that these elements are derived primarily from three sources: forcing depends on both physical and chemical properties (1) regional crustal emissions, including possible resuspen- of aerosols, including particle size and chemical composi- sion of soils containing biogenic P, (2) long-range transport, tion (Pilinis et al., 1995). -

Minimal Geological Methane Emissions During the Younger Dryas-Preboreal Abrupt Warming Event
UC San Diego UC San Diego Previously Published Works Title Minimal geological methane emissions during the Younger Dryas-Preboreal abrupt warming event. Permalink https://escholarship.org/uc/item/1j0249ms Journal Nature, 548(7668) ISSN 0028-0836 Authors Petrenko, Vasilii V Smith, Andrew M Schaefer, Hinrich et al. Publication Date 2017-08-01 DOI 10.1038/nature23316 Peer reviewed eScholarship.org Powered by the California Digital Library University of California LETTER doi:10.1038/nature23316 Minimal geological methane emissions during the Younger Dryas–Preboreal abrupt warming event Vasilii V. Petrenko1, Andrew M. Smith2, Hinrich Schaefer3, Katja Riedel3, Edward Brook4, Daniel Baggenstos5,6, Christina Harth5, Quan Hua2, Christo Buizert4, Adrian Schilt4, Xavier Fain7, Logan Mitchell4,8, Thomas Bauska4,9, Anais Orsi5,10, Ray F. Weiss5 & Jeffrey P. Severinghaus5 Methane (CH4) is a powerful greenhouse gas and plays a key part atmosphere can only produce combined estimates of natural geological in global atmospheric chemistry. Natural geological emissions and anthropogenic fossil CH4 emissions (refs 2, 12). (fossil methane vented naturally from marine and terrestrial Polar ice contains samples of the preindustrial atmosphere and seeps and mud volcanoes) are thought to contribute around offers the opportunity to quantify geological CH4 in the absence of 52 teragrams of methane per year to the global methane source, anthropogenic fossil CH4. A recent study used a combination of revised 13 13 about 10 per cent of the total, but both bottom-up methods source δ C isotopic signatures and published ice core δ CH4 data to 1 −1 2 (measuring emissions) and top-down approaches (measuring estimate natural geological CH4 at 51 ± 20 Tg CH4 yr (1σ range) , atmospheric mole fractions and isotopes)2 for constraining these in agreement with the bottom-up assessment of ref. -

Office of Polar Programs
DEVELOPMENT AND IMPLEMENTATION OF SURFACE TRAVERSE CAPABILITIES IN ANTARCTICA COMPREHENSIVE ENVIRONMENTAL EVALUATION DRAFT (15 January 2004) FINAL (30 August 2004) National Science Foundation 4201 Wilson Boulevard Arlington, Virginia 22230 DEVELOPMENT AND IMPLEMENTATION OF SURFACE TRAVERSE CAPABILITIES IN ANTARCTICA FINAL COMPREHENSIVE ENVIRONMENTAL EVALUATION TABLE OF CONTENTS 1.0 INTRODUCTION....................................................................................................................1-1 1.1 Purpose.......................................................................................................................................1-1 1.2 Comprehensive Environmental Evaluation (CEE) Process .......................................................1-1 1.3 Document Organization .............................................................................................................1-2 2.0 BACKGROUND OF SURFACE TRAVERSES IN ANTARCTICA..................................2-1 2.1 Introduction ................................................................................................................................2-1 2.2 Re-supply Traverses...................................................................................................................2-1 2.3 Scientific Traverses and Surface-Based Surveys .......................................................................2-5 3.0 ALTERNATIVES ....................................................................................................................3-1 -

Sam White the Real Little Ice Age Between C.1300 and C.1850 A.D
Journal of Interdisciplinary History, xliv:3 (Winter, 2014), 327–352. THE REAL LITTLE ICE AGE Sam White The Real Little Ice Age Between c.1300 and c.1850 a.d. the world became, on average, slightly but signiªcantly colder. The change varied over time and space, and its causes remain un- certain. Nevertheless, this cooling constitutes a meaningful climate event, with signiªcant historical consequences. Both the cooling trend and its effects on humans appear to have been particularly Downloaded from http://direct.mit.edu/jinh/article-pdf/44/3/327/1706251/jinh_a_00574.pdf by guest on 28 September 2021 acute from the late sixteenth to the late seventeenth century in much of the Northern Hemisphere. This article explains why climatologists and historians are conªdent that these changes occurred. On close examination, the objections raised in this issue of the journal by Kelly and Ó Gráda turn out to be entirely unfounded. The proxy data for early mod- ern global cooling (such as tree rings and ice cores) are robust, and written weather descriptions and observations of physical phenom- ena (such as glacial movements and river freezings) by and large of- fer independent conªrmation. Kelly and Ó Gráda’s proposed alter- native measures of climate and climate change suffer from serious ºaws. As we review the evidence and refute their criticisms, it will become clear just how solid the case for the Little Ice Age (lia) has become. the case for the little ice age The evidence for early modern global cooling comes, ªrst and foremost, from extensive research into physical proxies, including ice cores, tree rings, corals, and speleothems (stalagmites and stalactites). -

Revised Records of Atmospheric Trace Gases CO2, CH4, N2O, and Δ13c
Earth Syst. Sci. Data, 11, 473–492, 2019 https://doi.org/10.5194/essd-11-473-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Revised records of atmospheric trace gases 13 CO2, CH4, N2O, and δ C-CO2 over the last 2000 years from Law Dome, Antarctica Mauro Rubino1,2, David M. Etheridge2, David P. Thornton2, Russell Howden2, Colin E. Allison2, Roger J. Francey2, Ray L. Langenfelds2, L. Paul Steele2, Cathy M. Trudinger2, Darren A. Spencer2, Mark A. J. Curran3,4, Tas D. van Ommen3,4, and Andrew M. Smith5 1School of Geography, Geology and the Environment, Keele University, Keele, ST5 5BG, UK 2Climate Science Centre, CSIRO Oceans and Atmosphere, Aspendale, Victoria, 3195, Australia 3Australian Antarctic Division, Channel Highway, Kingston, Tasmania, 7050, Australia 4Antarctic Climate and Ecosystems Cooperative Research Centre, University of Tasmania, Private Bag 80, Hobart, Tasmania, 7005, Australia 5Australian Nuclear Science and Technology Organisation (ANSTO), Locked Bag 2001, Kirrawee, NSW 2232, Australia Correspondence: Mauro Rubino ([email protected]) and David M. Etheridge ([email protected]) Received: 28 November 2018 – Discussion started: 14 December 2018 Revised: 12 March 2019 – Accepted: 24 March 2019 – Published: 11 April 2019 Abstract. Ice core records of the major atmospheric greenhouse gases (CO2, CH4,N2O) and their isotopo- logues covering recent centuries provide evidence of biogeochemical variations during the Late Holocene and pre-industrial periods and over the transition to the industrial period. These records come from a number of ice core and firn air sites and have been measured in several laboratories around the world and show com- mon features but also unresolved differences. -
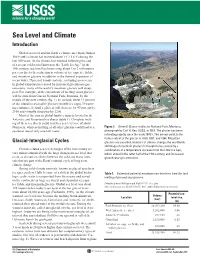
Sea Level and Climate Introduction
Sea Level and Climate Introduction Global sea level and the Earth’s climate are closely linked. The Earth’s climate has warmed about 1°C (1.8°F) during the last 100 years. As the climate has warmed following the end of a recent cold period known as the “Little Ice Age” in the 19th century, sea level has been rising about 1 to 2 millimeters per year due to the reduction in volume of ice caps, ice fields, and mountain glaciers in addition to the thermal expansion of ocean water. If present trends continue, including an increase in global temperatures caused by increased greenhouse-gas emissions, many of the world’s mountain glaciers will disap- pear. For example, at the current rate of melting, most glaciers will be gone from Glacier National Park, Montana, by the middle of the next century (fig. 1). In Iceland, about 11 percent of the island is covered by glaciers (mostly ice caps). If warm- ing continues, Iceland’s glaciers will decrease by 40 percent by 2100 and virtually disappear by 2200. Most of the current global land ice mass is located in the Antarctic and Greenland ice sheets (table 1). Complete melt- ing of these ice sheets could lead to a sea-level rise of about 80 meters, whereas melting of all other glaciers could lead to a Figure 1. Grinnell Glacier in Glacier National Park, Montana; sea-level rise of only one-half meter. photograph by Carl H. Key, USGS, in 1981. The glacier has been retreating rapidly since the early 1900’s. -

Calving Processes and the Dynamics of Calving Glaciers ⁎ Douglas I
Earth-Science Reviews 82 (2007) 143–179 www.elsevier.com/locate/earscirev Calving processes and the dynamics of calving glaciers ⁎ Douglas I. Benn a,b, , Charles R. Warren a, Ruth H. Mottram a a School of Geography and Geosciences, University of St Andrews, KY16 9AL, UK b The University Centre in Svalbard, PO Box 156, N-9171 Longyearbyen, Norway Received 26 October 2006; accepted 13 February 2007 Available online 27 February 2007 Abstract Calving of icebergs is an important component of mass loss from the polar ice sheets and glaciers in many parts of the world. Calving rates can increase dramatically in response to increases in velocity and/or retreat of the glacier margin, with important implications for sea level change. Despite their importance, calving and related dynamic processes are poorly represented in the current generation of ice sheet models. This is largely because understanding the ‘calving problem’ involves several other long-standing problems in glaciology, combined with the difficulties and dangers of field data collection. In this paper, we systematically review different aspects of the calving problem, and outline a new framework for representing calving processes in ice sheet models. We define a hierarchy of calving processes, to distinguish those that exert a fundamental control on the position of the ice margin from more localised processes responsible for individual calving events. The first-order control on calving is the strain rate arising from spatial variations in velocity (particularly sliding speed), which determines the location and depth of surface crevasses. Superimposed on this first-order process are second-order processes that can further erode the ice margin. -

Surface Characterisation of the Dome Concordia Area (Antarctica) As a Potential Satellite Calibration Site, Using Spot 4/Vegetation Instrument
Remote Sensing of Environment 89 (2004) 83–94 www.elsevier.com/locate/rse Surface characterisation of the Dome Concordia area (Antarctica) as a potential satellite calibration site, using Spot 4/Vegetation instrument Delphine Sixa, Michel Filya,*,Se´verine Alvainb, Patrice Henryc, Jean-Pierre Benoista a Laboratoire de Glaciologie et Ge´ophysique de l’Environnemlent, CNRS/UJF, 54 rue Molie`re, BP 96, 38 402 Saint Martin d’He`res Cedex, France b Laboratoire des Sciences du Climat et de l’Environnement, CEA/CNRS, L’Orme des Merisiers, CE Saclay, Bat. 709, 91 191 Gif-sur-Yvette, France c Centre National d’Etudes Spatiales, Division Qualite´ et Traitement de l’Imagerie Spatiale, Capteurs Grands Champs, 18 Avenue Edouard Belin, 33 401 Toulouse Cedex 4, France Received 7 July 2003; received in revised form 10 October 2003; accepted 14 October 2003 Abstract A good calibration of satellite sensors is necessary to derive reliable quantitative measurements of the surface parameters or to compare data obtained from different sensors. In this study, the snow surface of the high plateau of the East Antarctic ice sheet, particularly the Dome C area (75jS, 123jE), is used first to test the quality of this site as a ground calibration target and then to determine the inter-annual drift in the sensitivity of the VEGETATION sensor, onboard the SPOT4 satellite. Dome C area has many good calibration site characteristics: The site is very flat and extremely homogeneous (only snow), there is little wind and a very small snow accumulation rate and therefore a small temporal variability, the elevation is 3200 m and the atmosphere is very clear most of the time. -

Comparison of Remote Sensing Extraction Methods for Glacier Firn Line- Considering Urumqi Glacier No.1 As the Experimental Area
E3S Web of Conferences 218, 04024 (2020) https://doi.org/10.1051/e3sconf/202021804024 ISEESE 2020 Comparison of remote sensing extraction methods for glacier firn line- considering Urumqi Glacier No.1 as the experimental area YANJUN ZHAO1, JUN ZHAO1, XIAOYING YUE2and YANQIANG WANG1 1College of Geography and Environmental Science, Northwest Normal University, Lanzhou, China 2State Key Laboratory of Cryospheric Sciences, Northwest Institute of Eco-Environment and Resources/Tien Shan Glaciological Station, Chinese Academy of Sciences, Lanzhou, China Abstract. In mid-latitude glaciers, the altitude of the snowline at the end of the ablating season can be used to indicate the equilibrium line, which can be used as an approximation for it. In this paper, Urumqi Glacier No.1 was selected as the experimental area while Landsat TM/ETM+/OLI images were used to analyze and compare the accuracy as well as applicability of the visual interpretation, Normalized Difference Snow Index, single-band threshold and albedo remote sensing inversion methods for the extraction of the firn lines. The results show that the visual interpretation and the albedo remote sensing inversion methods have strong adaptability, alonger with the high accuracy of the extracted firn line while it is followed by the Normalized Difference Snow Index and the single-band threshold methods. In the year with extremely negative mass balance, the altitude deviation of the firn line extracted by different methods is increased. Except for the years with extremely negative mass balance, the altitude of the firn line at the end of the ablating season has a good indication for the altitude of the balance line. -

A Globally Complete Inventory of Glaciers
Journal of Glaciology, Vol. 60, No. 221, 2014 doi: 10.3189/2014JoG13J176 537 The Randolph Glacier Inventory: a globally complete inventory of glaciers W. Tad PFEFFER,1 Anthony A. ARENDT,2 Andrew BLISS,2 Tobias BOLCH,3,4 J. Graham COGLEY,5 Alex S. GARDNER,6 Jon-Ove HAGEN,7 Regine HOCK,2,8 Georg KASER,9 Christian KIENHOLZ,2 Evan S. MILES,10 Geir MOHOLDT,11 Nico MOÈ LG,3 Frank PAUL,3 Valentina RADICÂ ,12 Philipp RASTNER,3 Bruce H. RAUP,13 Justin RICH,2 Martin J. SHARP,14 THE RANDOLPH CONSORTIUM15 1Institute of Arctic and Alpine Research, University of Colorado, Boulder, CO, USA 2Geophysical Institute, University of Alaska Fairbanks, Fairbanks, AK, USA 3Department of Geography, University of ZuÈrich, ZuÈrich, Switzerland 4Institute for Cartography, Technische UniversitaÈt Dresden, Dresden, Germany 5Department of Geography, Trent University, Peterborough, Ontario, Canada E-mail: [email protected] 6Graduate School of Geography, Clark University, Worcester, MA, USA 7Department of Geosciences, University of Oslo, Oslo, Norway 8Department of Earth Sciences, Uppsala University, Uppsala, Sweden 9Institute of Meteorology and Geophysics, University of Innsbruck, Innsbruck, Austria 10Scott Polar Research Institute, University of Cambridge, Cambridge, UK 11Institute of Geophysics and Planetary Physics, Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA, USA 12Department of Earth, Ocean and Atmospheric Sciences, University of British Columbia, Vancouver, British Columbia, Canada 13National Snow and Ice Data Center, University of Colorado, Boulder, CO, USA 14Department of Earth and Atmospheric Sciences, University of Alberta, Edmonton, Alberta, Canada 15A complete list of Consortium authors is in the Appendix ABSTRACT. The Randolph Glacier Inventory (RGI) is a globally complete collection of digital outlines of glaciers, excluding the ice sheets, developed to meet the needs of the Fifth Assessment of the Intergovernmental Panel on Climate Change for estimates of past and future mass balance.