New Hampshire Wildlife Action Plan Appendix a Mammals-1 Appendix A: Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
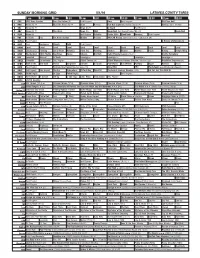
Sunday Morning Grid 5/1/16 Latimes.Com/Tv Times
SUNDAY MORNING GRID 5/1/16 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Sunday Face the Nation (N) Paid Program Boss Paid Program PGA Tour Golf 4 NBC News (N) Å Meet the Press (N) Å News Rescue Red Bull Signature Series (Taped) Å Hockey: Blues at Stars 5 CW News (N) Å News (N) Å In Touch Paid Program 7 ABC News (N) Å This Week News (N) NBA Basketball First Round: Teams TBA. (N) Basketball 9 KCAL News (N) Joel Osteen Schuller Pastor Mike Woodlands Amazing Paid Program 11 FOX In Touch Paid Fox News Sunday Midday Prerace NASCAR Racing Sprint Cup Series: GEICO 500. (N) 13 MyNet Paid Program A History of Violence (R) 18 KSCI Paid Hormones Church Faith Paid Program 22 KWHY Local Local Local Local Local Local Local Local Local Local Local Local 24 KVCR Landscapes Painting Joy of Paint Wyland’s Paint This Painting Kitchen Mexico Martha Pépin Baking Simply Ming 28 KCET Wunderkind 1001 Nights Bug Bites Space Edisons Biz Kid$ Celtic Thunder Legacy (TVG) Å Soulful Symphony 30 ION Jeremiah Youssef In Touch Leverage Å Leverage Å Leverage Å Leverage Å 34 KMEX Conexión En contacto Paid Program Fútbol Central (N) Fútbol Mexicano Primera División: Toluca vs Azul República Deportiva (N) 40 KTBN Walk in the Win Walk Prince Carpenter Schuller In Touch PowerPoint It Is Written Pathway Super Kelinda Jesse 46 KFTR Paid Program Formula One Racing Russian Grand Prix. -

Ontario Species at Risk Evaluation Report for Tri-Colored Bat
Ontario Species at Risk Evaluation Report for Tri-colored Bat (Perimyotis subflavus) Committee on the Status of Species at Risk in Ontario (COSSARO) Assessed by COSSARO as Endangered June, 2015 Final Pipistrelle de l’Est (Perimyotis subflavus) La pipistrelle de l’Est (Perimyotis subflavus) est l’une des plus petites chauves-souris en Amérique du Nord. Environ 10 p. 100 de son aire de répartition mondiale se situe au Canada (en Ontario, au Québec, au Nouveau-Brunswick et en Nouvelle-Écosse) et elle est considérée rare dans la majeure partie de son aire de répartition canadienne. En Ontario, elle est considérée peu courante, bien que la taille des populations ne soit pas bien connue. La pipistrelle de l’Est se nourrit d’insectes. Elle s’alimente au-dessus de l’eau, le long des cours d’eau ainsi qu’à la lisière des forêts; elle évite généralement les grands champs ouverts ou les zones de coupe à blanc. À l’automne, les chauves-souris reviennent aux gîtes d’hibernation, qui peuvent être à des centaines de kilomètres de distance de leurs sites d’été. Elles s’agglutinent près de l’entrée, elles s’accouplent, puis elles pénètrent dans ce gîte d’hibernation ou elles se déplacent vers un gîte différent pour y passer l’hiver. La femelle produit un ou deux petits par année après l’âge d’un an et la longévité maximale consignée est de 15 ans. La principale menace qui pèse sur la pipistrelle de l’Est est une maladie appelée le syndrome du museau blanc (SMB), qui est causé par l’introduction du champignon Pseudogymnoascus destructans. -

Fungal Sampling of a Maternity Roost of Big Brown Bats (Eptesicus Fuscus) on the Baca National Wildlife Refuge
Fungal sampling of a maternity roost of Big Brown Bats (Eptesicus fuscus) on the Baca National Wildlife Refuge. Erin M Lehmer, Stephen Fenster & Kirk Navo Background The initial research was focused on sampling fungal community diversity on the migratory Mexican free-tailed bat (Tadarida brasiliensis) population from the Orient Mine upon arrival and prior to departure from Colorado. However, in June 2015 because of cold spring temperatures and higher than average precipitation, arrival of the free-tailed population was delayed, and we were unable to capture bats after repeated sampling efforts. Because of these failed efforts, it was decided to move to the nearby Baca National Wildlife Refuge in an attempt to capture resident (i.e. non-migratory) bats, using a stacked mist net system. During the single night of sampling at the Baca NWR, we captured 32 adult female big brown bats (Eptesicus fuscus) from a single maternity roost located in the attic of an abandoned outbuilding on the refuge property. These bats were processed in the same manner that we had processed the free-tailed bats in previous seasons; after capture, they were weighed, sex and reproductive condition were determined, and forearm lengths were measured. Fungal spores were collected by swabbing the wing membranes and dorsal and ventral fur with sterile cotton swabs dipped in sterile water. During routine processing of the fungal spores (i.e. culturing, PCR and DNA sequence barcoding analysis), we determined that 2 of the samples were a very close genetic match to P. destructans based on sequence alignment data of the internal transcribed spacer (ITS) region of the genome. -

<I>Geomyces Destructans</I> Sp. Nov. Associated with Bat White-Nose
MYCOTAXON Volume 108, pp. 147–154 April–June 2009 Geomyces destructans sp. nov. associated with bat white-nose syndrome A. Gargas1, M.T. Trest2, M. Christensen3 T.J. Volk4 & D.S. Blehert5* [email protected] Symbiology LLC Middleton, WI 53562 USA [email protected] Department of Botany, University of Wisconsin — Madison Birge Hall, 430 Lincoln Drive, Madison, WI 53706 USA [email protected] 1713 Frisch Road, Madison, WI 53711 USA [email protected] Department of Biology, University of Wisconsin — La Crosse 3024 Crowley Hall, La Crosse, WI 54601 USA [email protected] U.S. Geological Survey — National Wildlife Health Center 6006 Schroeder Road, Madison, WI 53711 USA Abstract — We describe and illustrate the new species Geomyces destructans. Bats infected with this fungus present with powdery conidia and hyphae on their muzzles, wing membranes, and/or pinnae, leading to description of the accompanying disease as white-nose syndrome, a cause of widespread mortality among hibernating bats in the northeastern US. Based on rRNA gene sequence (ITS and SSU) characters the fungus is placed in the genus Geomyces, yet its distinctive asymmetrically curved conidia are unlike those of any described Geomyces species. Key words — Ascomycota, Helotiales, Pseudogymnoascus, psychrophilic, systematics Introduction Bat white-nose syndrome (WNS) was first documented in a photograph taken at Howes Cave, 52 km west of Albany, NY USA during winter, 2006 (Blehert et al. 2009). As of March 2009, WNS has been confirmed by gross and histologic examination of bats at caves and mines in Massachusetts, New Jersey, Vermont, West Virginia, New Hampshire, Connecticut, Virginia, and Pennsylvania. -

Anne Sexton and Sylvia Plath from a Kristevan Perspective
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by OpenGrey Repository Transforming the Law of One: Anne Sexton and Sylvia Plath from a Kristevan Perspective A thesis submitted for the degree of Doctor of Philosophy By Areen Ghazi Khalifeh School of Arts, Brunel University November 2010 ii Abstract A recent trend in the study of Anne Sexton and Sylvia Plath often dissociates Confessional poetry from the subject of the writer and her biography, claiming that the artist is in full control of her work and that her art does not have naïve mimetic qualities. However, this study proposes that subjective attributes, namely negativity and abjection, enable a powerful transformative dialectic. Specifically, it demonstrates that an emphasis on the subjective can help manifest the process of transgressing the law of One. The law of One asserts a patriarchal, monotheistic law as a social closed system and can be opposed to the bodily drives and its open dynamism. This project asserts that unique, creative voices are derived from that which is individual and personal and thus, readings of Confessional poetry are in fact best served by acknowledgment of the subjective. In order to stress the subject of the artist in Confessionalism, this study employed a psychoanalytical Kristevan approach. This enables consideration of the subject not only in terms of the straightforward narration of her life, but also in relation to her poetic language and the process of creativity where instinctual drives are at work. This study further applies a feminist reading to the subject‘s poetic language and its ability to transgress the law, not necessarily in the political, macrocosmic sense of the word, but rather on the microcosmic, subjective level. -

25 Chrysosporium
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universidade do Minho: RepositoriUM 25 Chrysosporium Dongyou Liu and R.R.M. Paterson contents 25.1 Introduction ..................................................................................................................................................................... 197 25.1.1 Classification and Morphology ............................................................................................................................ 197 25.1.2 Clinical Features .................................................................................................................................................. 198 25.1.3 Diagnosis ............................................................................................................................................................. 199 25.2 Methods ........................................................................................................................................................................... 199 25.2.1 Sample Preparation .............................................................................................................................................. 199 25.2.2 Detection Procedures ........................................................................................................................................... 199 25.3 Conclusion .......................................................................................................................................................................200 -

Best Management Practices for the New England Cottontail - New York
Best Management Practices For the New England Cottontail New York Specific challenges Invasive shrubs Heathlands Canopy Retention Eastern cottontails Statement of Purpose Populations of species residing at the edge of their range are exposed to novel environments and stressors that may affect their response to management. The impacts of eastern cottontails and the prevalence of invasive shrubs have been recognized as factors limiting New England cottontail populations at the edge of their range in New York State. Here, canopy closure, heathlands, and invasive shrubs may also play a large role in providing habitat and mitigating the negative impacts of competition with the eastern cottontail. This document is meant to serve as a technical guide for managers working to restore or create New England cottontail habitat in the face of these challenges. Recent work suggests current management practices may be ineffective or even harmful when the impacts of invasive shrubs and eastern cottontails are not considered in forest management decision- making. These guidelines provide background information and updated recommendations derived from recent and ongoing research on New England cottontails for use in developing site specific forest management plans. While we use New York specific examples, many of these challenges we discuss, such as management of New England cottontails in the presence of eastern cottontails, are rapidly becoming a range-wide concern. The guidance outlined herein is adaptable to similar habitat in New England. Prepared by: Amanda Cheeseman PhD. and Jonathan Cohen PhD from the State University of New York College of Environmental Science and Forestry in partnership with the New York State Department of Environmental Conservation. -

Biosafety Measures for Working with Pseudogymnoascus Destructans in the Laboratory and Use Or Storage of Potentially Contaminated Materials
Biosafety Measures for Working with Pseudogymnoascus destructans in the Laboratory and Use or Storage of Potentially Contaminated Materials The emergent disease of bats, white-nose syndrome (WNS), has caused one of the most precipitous declines documented among North American wildlife. This disease is caused by the recently described fungal pathogen Pseudogymnoascus (formerly Geomyces) destructans. Because of the grave threat this fungus presents to populations of hibernating bats, precautions must be taken when working with P. destructans in the laboratory to prevent accidental release of the fungus into the environment. The following guidelines apply to all members of the WNS Diagnostic Laboratory Network. Other laboratories that work with P. destructans or that maintain specimens that may harbor viable fungus may use these guidelines to develop appropriate institutional standards for preventing accidental release of a Biosafety Level-2 (BSL-2) fungal pathogen of animals. Based upon biological risk assessment, work in the laboratory with viable P. destructans should be restricted to BSL-2 or higher. Adherence to this guidance will ensure that in accordance with the manual Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition, appropriate procedures, mechanical controls, and containment equipment are in place to facilitate biosecure work with the fungus and to prevent accidental release. Use of a biosafety cabinet. Fungi such as P. destructans readily produce aerosolizeable, environmentally resistant, and long-lived spores (reproductive structures). Therefore, all manipulations of potentially viable P. destructans (fungal cultures, carcasses, unfixed tissue samples, wing swabs, environmental samples, fungal tape lifts) in the laboratory should be restricted to a certified Class I or Class II biosafety cabinet. -
Wildlife in Your Young Forest.Pdf
WILDLIFE IN YOUR Young Forest 1 More Wildlife in Your Woods CREATE YOUNG FOREST AND ENJOY THE WILDLIFE IT ATTRACTS WHEN TO EXPECT DIFFERENT ANIMALS his guide presents some of the wildlife you may used to describe this dense, food-rich habitat are thickets, T see using your young forest as it grows following a shrublands, and early successional habitat. timber harvest or other management practice. As development has covered many acres, and as young The following lists focus on areas inhabited by the woodlands have matured to become older forest, the New England cottontail (Sylvilagus transitionalis), a rare amount of young forest available to wildlife has dwindled. native rabbit that lives in parts of New York east of the Having diverse wildlife requires having diverse habitats on Hudson River, and in parts of Connecticut, Rhode Island, the land, including some young forest. Massachusetts, southern New Hampshire, and southern Maine. In this region, conservationists and landowners In nature, young forest is created by floods, wildfires, storms, are carrying out projects to create the young forest and and beavers’ dam-building and feeding. To protect lives and shrubland that New England cottontails need to survive. property, we suppress floods, fires, and beaver activities. Such projects also help many other kinds of wildlife that Fortunately, we can use habitat management practices, use the same habitat. such as timber harvests, to mimic natural disturbance events and grow young forest in places where it will do the most Young forest provides abundant food and cover for insects, good. These habitat projects boost the amount of food reptiles, amphibians, birds, and mammals. -

Ericaceae Root Associated Fungi Revealed by Culturing and Culture – Independent Molecular Methods
a Ericaceae root associated fungi revealed by culturing and culture – independent molecular methods. by Damian S. Bougoure BSc (Hons) Thesis submitted in accordance with the requirements for the degree of Doctor of Philosophy Centre for Horticulture and Plant Sciences University of Western Sydney February 2006 2 ACKNOWLEDGEMENTS Although I am credited with writing this thesis there is a multitude of people that have contributed to its completion in ways other than hitting the letters on a keyboard and I would like to thank them here. Firstly I’d like to thank my supervisor, Professor John Cairney, whose knowledge and guidance was invaluable in steering me along the PhD path. The timing of John’s ‘motivational chats’ was uncanny and his patience particularly, during the writing stage, seemed limitless at times. I’d also like to thank the Australian government for granting me an Australian Postgraduate Award (APA) scholarship, Paul Worden from Macquarie University and the staff from the Millennium Institute at Westmead Hospital for performing DNA sequencing and the National Parks and Wildlife Service of New South Wales and Environmental Protection agency of Queensland for permission to collect the Ericaceae plants. Thankyou to Mary Gandini from James Cook University for showing me the path to a Rhododendron lochiae population through the thick North Queenland rainforest. Without her help and I’d still be pointing the GPS at the sky. Thankyou to the other people in the lab studying mycorrhizas including Catherine Hitchcock, Susan Chambers, Adrienne Williams and particularly Brigitte Bastias with whom I shared an office. Everyone mentioned was generally just as willing as I was to talk about matters other than mycorrhizas. -

Cottontail Story For
CottontailNew England’s © ANNE BROWN PHOTO Relict, opportunistic or soon-to-be endangered species, the New England cottontail has managed to keep a low profile. But it is in danger of disappearing from the woodlands of New Hampshire. BY JOHN A. LITVAITIS t one time or another, most of us have encountered a small state: Eastern and New England. And it is the New England Abrown bunny while out for a walk or while doing chores in cottontail that has our concern. Before I summarize the reasons for the backyard. If you’re a hunter, your experiences also may have that concern, let me give you a little background information. included walking through a brushy field hoping to kick up a In general appearance, New England cottontails are like rabbit or two for the stewpot. other North American rabbits. Smaller than Eastern cottontails, Hunters and naturalists in New Hampshire know that rabbits New England cottontails weigh just about 2 pounds. Brown and (cottontails) and snowshoe hares both occur in the state. In summer, a conspicuous white tail describe most rabbits. However, if you they’re often difficult to tell apart because they both have a brown look closely, you can find a few characteristics that can help you coat and usually don’t stand still long enough for us to get a good distinguish a New England from an Eastern cottontail. About look. In winter, however, the coat of a snowshoe hare turns white half of Eastern cottontails have a small white spot on their and that of a cottontail remains brown. -

2011 Annual Report of Center for Biological Diversity
2 011 ANNUAL REPORT CENTER FOR BIOLOGICAL DIVERSITY annual report photography (Cover) scarlet Hawaiian honeycreeper © Tom Ranker; (inside cover) Grand Canyon courtesy Flickr Commons/racoles; (p. 2) wolverine © Larry Master/masterimages.org; scarlet Hawaiian honeycreeper courtesy Flickr Commons/Ludovich Hirlimann; Miami blue butterfly © Jaret C. Daniels, McGuire Center for Lepidoptera Biodiversity; (p. 3) Pacific walruses courtesy USFWS; (p. 4) gray wolf courtesy Flickr Commons/dalliedee; (p. 6) thread-leaved brodiaea courtesy USFWS, Hawaiian monk seal courtesy Flickr Commons/Brian Russo; (p. 7) beluga whale courtesy Flickr Commons/ivan; (p. 8) Grand Canyon courtesy Flickr Commons/Paul Fundenburg; (p. 9) Center mascot Frostpaw and Barbara Kingsolver by the Center for Biological Diversity; (p. 10) ringed seal © John Moran; (p. 11) polar bear by Jason Molenda; (p. 12) San Joaquin kit fox © B. Moose Peterson; (p. 13) Laysan albatross courtesy USFWS; (p. 14) Florida panther courtesy Flickr Commons/Monica R; (p. 15) whooping crane courtesy Flickr Commons/ NaturesFan1266; (p. 16) California red-legged frog; flat-tailed horned lizard by Wendy Hodges; (p. 17) California condor courtesy Flickr Commons/DJMcCradey; (p. 18) 7 Billion and Counting Logo © Amy Harwood; (p. 19) caribou by John Nickles/USFWS; (p. 20) Seattle courtesy Flickr Commons/craterlover; (p. 21) Species Finder by the Center for Biological Diversity; (p. 22) steelhead trout courtesy Flickr Commons/sgrace; (p. 23) California spotted owl courtesy USFWS, (p. 24) loggerhead sea turtle courtesy Flickr Commons/Wendell Reed, leatherback sea turtle hatchling courtesy Flickr Commons/algaedoc Printed on 100% post-consumer recycled paper with solvent-free vegetable-based inks. Letter From the Director 2011 was an exciting year at the Center.