Bachmann Etal Suppinfo 20150402 Edits2016
Total Page:16
File Type:pdf, Size:1020Kb
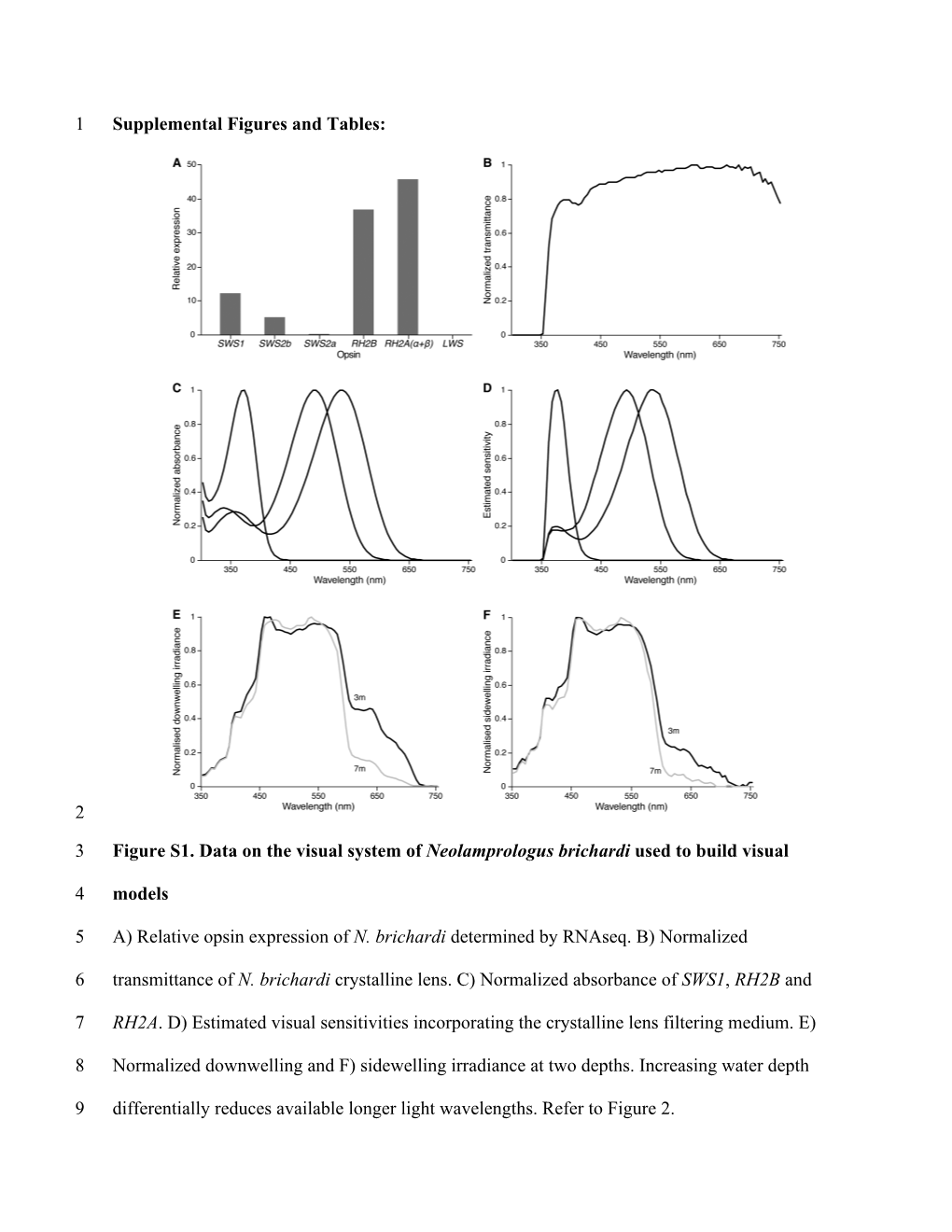
Load more
Recommended publications
-

Phylogeny of a Rapidly Evolving Clade: the Cichlid Fishes of Lake Malawi
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 5107–5110, April 1999 Evolution Phylogeny of a rapidly evolving clade: The cichlid fishes of Lake Malawi, East Africa (adaptive radiationysexual selectionyspeciationyamplified fragment length polymorphismylineage sorting) R. C. ALBERTSON,J.A.MARKERT,P.D.DANLEY, AND T. D. KOCHER† Department of Zoology and Program in Genetics, University of New Hampshire, Durham, NH 03824 Communicated by John C. Avise, University of Georgia, Athens, GA, March 12, 1999 (received for review December 17, 1998) ABSTRACT Lake Malawi contains a flock of >500 spe- sponsible for speciation, then we expect that sister taxa will cies of cichlid fish that have evolved from a common ancestor frequently differ in color pattern but not morphology. within the last million years. The rapid diversification of this Most attempts to determine the relationships among cichlid group has been attributed to morphological adaptation and to species have used morphological characters, which may be sexual selection, but the relative timing and importance of prone to convergence (8). Molecular sequences normally these mechanisms is not known. A phylogeny of the group provide the independent estimate of phylogeny needed to infer would help identify the role each mechanism has played in the evolutionary mechanisms. The Lake Malawi cichlids, however, evolution of the flock. Previous attempts to reconstruct the are speciating faster than alleles can become fixed within a relationships among these taxa using molecular methods have species (9, 10). The coalescence of mtDNA haplotypes found been frustrated by the persistence of ancestral polymorphisms within populations predates the origin of many species (11). In within species. -

Genome Sequences of Tropheus Moorii and Petrochromis Trewavasae, Two Eco‑Morphologically Divergent Cichlid Fshes Endemic to Lake Tanganyika C
www.nature.com/scientificreports OPEN Genome sequences of Tropheus moorii and Petrochromis trewavasae, two eco‑morphologically divergent cichlid fshes endemic to Lake Tanganyika C. Fischer1,2, S. Koblmüller1, C. Börger1, G. Michelitsch3, S. Trajanoski3, C. Schlötterer4, C. Guelly3, G. G. Thallinger2,5* & C. Sturmbauer1,5* With more than 1000 species, East African cichlid fshes represent the fastest and most species‑rich vertebrate radiation known, providing an ideal model to tackle molecular mechanisms underlying recurrent adaptive diversifcation. We add high‑quality genome reconstructions for two phylogenetic key species of a lineage that diverged about ~ 3–9 million years ago (mya), representing the earliest split of the so‑called modern haplochromines that seeded additional radiations such as those in Lake Malawi and Victoria. Along with the annotated genomes we analysed discriminating genomic features of the study species, each representing an extreme trophic morphology, one being an algae browser and the other an algae grazer. The genomes of Tropheus moorii (TM) and Petrochromis trewavasae (PT) comprise 911 and 918 Mbp with 40,300 and 39,600 predicted genes, respectively. Our DNA sequence data are based on 5 and 6 individuals of TM and PT, and the transcriptomic sequences of one individual per species and sex, respectively. Concerning variation, on average we observed 1 variant per 220 bp (interspecifc), and 1 variant per 2540 bp (PT vs PT)/1561 bp (TM vs TM) (intraspecifc). GO enrichment analysis of gene regions afected by variants revealed several candidates which may infuence phenotype modifcations related to facial and jaw morphology, such as genes belonging to the Hedgehog pathway (SHH, SMO, WNT9A) and the BMP and GLI families. -

Paternity of Subordinates Raises Cooperative Effort in Cichlids
Paternity of Subordinates Raises Cooperative Effort in Cichlids Rick Bruintjes1,2*, Danielle Bonfils1, Dik Heg1,3, Michael Taborsky1 1 Department of Behavioural Ecology, Institute of Ecology and Evolution, University of Bern, Hinterkappelen, Switzerland, 2 School of Biological Sciences, University of Bristol, Bristol, United Kingdom, 3 Institute of Social and Preventive Medicine (ISPM), University of Bern, Bern, Switzerland Abstract Background: In cooperative breeders, subordinates generally help a dominant breeding pair to raise offspring. Parentage studies have shown that in several species subordinates can participate in reproduction. This suggests an important role of direct fitness benefits for cooperation, particularly where groups contain unrelated subordinates. In this situation parentage should influence levels of cooperation. Here we combine parentage analyses and detailed behavioural observations in the field to study whether in the highly social cichlid Neolamprologus pulcher subordinates participate in reproduction and if so, whether and how this affects their cooperative care, controlling for the effect of kinship. Methodology/Principal Findings: We show that: (i) male subordinates gained paternity in 27.8% of all clutches and (ii) if they participated in reproduction, they sired on average 11.8% of young. Subordinate males sharing in reproduction showed more defence against experimentally presented egg predators compared to subordinates not participating in reproduction, and they tended to stay closer to the breeding shelter. No effects of relatedness between subordinates and dominants (to mid-parent, dominant female or dominant male) were detected on parentage and on helping behaviour. Conclusions/Significance: This is the first evidence in a cooperatively breeding fish species that the helping effort of male subordinates may depend on obtained paternity, which stresses the need to consider direct fitness benefits in evolutionary studies of helping behaviour. -

The Use of Different Sensory Modalities in Predator Discrimination
Behav Ecol Sociobiol (2017) 71: 143 DOI 10.1007/s00265-017-2371-8 ORIGINAL ARTICLE Smell or vision? The use of different sensory modalities in predator discrimination Stefan Fischer1,2,3 & Evelyne Oberhummer1 & Filipa Cunha-Saraiva1,4 & Nina Gerber1,5 & Barbara Taborsky1 Received: 6 November 2016 /Revised: 19 August 2017 /Accepted: 28 August 2017 /Published online: 8 September 2017 # The Author(s) 2017. This article is an open access publication Abstract to a standardized threat stimulus combined with either preda- Theory predicts that animals should adjust their escape re- tor or non-predator cues presented either visually or chemical- sponses to the perceived predation risk. The information ani- ly. We predicted that flight responses towards a threat stimulus mals obtain about potential predation risk may differ qualita- are more pronounced if cues of dangerous rather than harm- tively depending on the sensory modality by which a cue is less heterospecifics are presented and that N. pulcher,beingan perceived. For instance, olfactory cues may reveal better in- aquatic species, relies more on olfaction when discriminating formation about the presence or absence of threats, whereas between dangerous and harmless heterospecifics. N. pulcher visual information can reliably transmit the position and po- responded faster to the threat stimulus, reached a refuge faster tential attack distance of a predator. While this suggests a and entered a refuge more likely when predator cues were differential use of information perceived through the two sen- perceived. Unexpectedly, the sensory modality used to per- sory channels, the relative importance of visual vs. olfactory ceive the cues did not affect the escape response or the dura- cues when distinguishing between different predation threats tion of the recovery phase. -

Effects of Increased Temperature And
EFFECTS OF INCREASED TEMPERATURE AND DECREASED FOOD QUALITY ON METABOLISM AND GROWTH OF AN ALGIVOROUS CICHLID, TROPHEUS DUBOISI, AND EFFECT OF FOOD HABIT ON THE FIELD METABOLISM OF AFRICAN CICHLIDS A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By LESLEY YU-JUNG KIM B.S., The Ohio State University, 2011 2014 Wright State University WRIGHT STATE UNIVERSITY GRADUATE SCHOOL September 2, 2014 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Lesley Yu-Jung Kim ENTITLED Effect of Increased Temperature and Decreased Food Quality on Metabolism and Growth of an Algivorous cichlid, Tropheus duboisi, and Effect of Food Habit on the Field Metabolism of African Cichlids BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science. __________________________ Yvonne Vadeboncoeur, Ph.D. Thesis Director __________________________ David Goldstein, Ph.D. Chair, Department of Biological Sciences Committee on Final Examination __________________________ Yvonne Vadeboncoeur, Ph.D. __________________________ Lynn Hartzler, Ph.D. __________________________ Volker Bahn, Ph.D. __________________________ Robert E. W. Fyffe, Ph.D. Vice President for Research and Dean of the Graduate School ABSTRACT Kim, Lesley Yu-Jung. M.S. Department of Biological Sciences, Wright State University, 2014. Effect of increased temperature and decreased food quality on metabolism and growth of an algivorous cichlid, Tropheus duboisi, and effect of food habit on the field metabolism of African Cichlids. The metabolic rate of an organism is influenced by mass, temperature, and diet. Climate change is anticipated to increase ambient temperatures of aquatic systems and decrease the quality of food available to algivorous fish. -

Neolamprologus Brichardi, a Singular Tanganyika Cichlid Text & Photos: José María Cid Ruiz
Neolamprologus brichardi, a singular Tanganyika cichlid Text & Photos: José María Cid Ruiz This species of attractive appearance and unique parental behavior, is one of the many endemic species that make the Lake Tanganyika (East Africa, originated 3-7 million years ago), a unique ecosystem with excep- tional biodiversity, such as is credited by 250 cichlid species and other 150 fish species from different families that inhabit its waters. As is well known, Neolamprologus brichardi1 (Poll,1974), was initially descri- bed as a subspecies of Lamprologus savoryi (L.savoryi elongatus) (Trewavas & Poll, 1952). The species has a laterally compressed body, which together with its long caudal in the form of "lyre", conveys an impression of great slender- ness. It's really a beautiful cichlid, with a uniform beige color on which stands out a peculiar drawing behind and below the eye, consisting of two black spots and a third spot in yellow-orange. It also highlights a metallic blue iridescence under the eye and at the edge of the fins. In the literature it is cited with sizes Averaging the various references consulted, we would consider a temperature in up to 10 cm, but generally, more developed adult males do not exceed the 8-9 shallow waters of 25-26 ° C. The pH in the majority of sample points has a value cm and females always measure something less. of 8.4 but there are areas with measurements of 9.2. Total hardness is moved in a range of GH: 7-11°d, while carbonate hardness ranges KH: 15 to 18°d. -

Testing the Potential of Environmental DNA Methods for Surveying Lake Tanganyika's Highly Diverse Fish Communities Christopher J
Testing the potential of environmental DNA methods for surveying Lake Tanganyika's highly diverse fish communities Christopher James Doble A thesis submitted for the degree of Doctor of Philosophy Department of Genetics, Evolution and Environment University College London April 2020 1 Declaration I, Christopher James Doble, confirm the work presented in this thesis is my own. Where information has been derived from other sources, I confirm this has been indicated in the thesis. Christopher James Doble Date: 27/04/2020 2 Statement of authorship I planned and undertook fieldwork to the Kigoma region of Lake Tanganyika, Tanzania in 2016 and 2017. This included obtaining research permits, collecting environmental DNA samples and undertaking fish community visual survey data used in Chapters three and four. For Chapter two, cichlid reference database sequences were sequenced by Walter Salzburger’s research group at the University of Basel. I extracted required regions from mitochondrial genome alignments during a visit to Walter’s research group. Other reference sequences were obtained by Sanger sequencing. I undertook the DNA extractions and PCR amplifications for all samples, with the clean-up and sequencing undertaken by the UCL Sequencing facility. I undertook the method development, DNA extractions, PCR amplifications and library preparations for each of the next generation sequencing runs in Chapters three and four at the NERC Biomolecular Analysis Facility Sheffield. Following training by Helen Hipperson at the NERC Biomolecular Analysis Facility in Sheffield, I undertook the bioinformatic analysis of sequence data in Chapters three and four. I also carried out all the data analysis within each chapter. Chapters two, three and parts of four have formed a manuscript recently published in Environmental DNA (Doble et al. -

BMC Evolutionary Biology Biomed Central
BMC Evolutionary Biology BioMed Central Research article Open Access Mitogenomic evaluation of the historical biogeography of cichlids toward reliable dating of teleostean divergences Yoichiro Azuma1, Yoshinori Kumazawa*2,3, Masaki Miya4, Kohji Mabuchi1 and Mutsumi Nishida1 Address: 1Ocean Research Institute, The University of Tokyo, 1-15-1 Minamidai, Nakano-ku, Tokyo 164-8639, Japan, 2Division of Material Science and Biological Science, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8602, Japan, 3Department of Information and Biological Sciences, Graduate School of Natural Sciences, Nagoya City University, 1 Yamanohata, Mizuho-cho, Mizuho-ku, Nagoya 467-8501, Japan and 4Department of Zoology, Natural History Museum and Institute, Chiba, 955-2 Aoba-cho, Chuo-ku, Chiba 260-8682, Japan Email: Yoichiro Azuma - [email protected]; Yoshinori Kumazawa* - [email protected]; Masaki Miya - [email protected]; Kohji Mabuchi - [email protected]; Mutsumi Nishida - [email protected] * Corresponding author Published: 23 July 2008 Received: 18 March 2008 Accepted: 23 July 2008 BMC Evolutionary Biology 2008, 8:215 doi:10.1186/1471-2148-8-215 This article is available from: http://www.biomedcentral.com/1471-2148/8/215 © 2008 Azuma et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Recent advances in DNA sequencing and computation offer the opportunity for reliable estimates of divergence times between organisms based on molecular data. -

Lunar and Diurnal Cycles in Reproductive Physiology and Behavior in a Natural Population of Cooperatively Breeding Fish J
Journal of Zoology Journal of Zoology. Print ISSN 0952-8369 Lunar and diurnal cycles in reproductive physiology and behavior in a natural population of cooperatively breeding fish J. K. Desjardins1,2, J. L. Fitzpatrick3,4, K. A. Stiver5,2, G. J. Van Der Kraak6 & S. Balshine2 1 Department of Biology, Stanford University, Stanford, CA, USA 2 Animal Behaviour Group, Department of Psychology, Neuroscience & Behaviour, McMaster University, Hamilton, ON, Canada 3 Centre for Evolutionary Biology, University of Western Australia, Crawley, Australia 4 Department of Biology, McMaster University, Hamilton, ON, Canada 5 Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT, USA 6 College of Biological Sciences, University of Guelph, Guelph, ON, Canada Keywords Abstract Lake Tanganyika; lunar phase; Neolamprologus pulcher; sperm swimming Natural environmental periodicity that occurs on both the small scale like day speed; cichlidae; testosterone; length, or larger scale like lunar light can provide animals with valuable informa- 11-ketotestosterone. tion about resource availability and predation risk. Such environmental cycles are often linked to the timing of reproduction. Here, using the circulating androgen Correspondence concentrations, gonadal investment patterns and detailed behavioral observations Julie K. Desjardins, Department of Biology, we show that wild populations of the group-living cichlid, Neolamprologus pulcher 371 Serra Mall, Stanford University, from Lake Tanganyika, have marked diurnal differences in behavior -

Download (1147Kb)
Supplementary Figure 1 | Phylogeny of East African cichlids based on a new multi- marker dataset. (a) Bayesian inference phylogeny with MrBayes. (b) Maximum likelihood phylogeny with GARLI and 500 bootstrap replicates. While most of the branches are supported with high posterior probabilities (a) and bootstrap values (b), the phylogenetic relationships among the more ancestral haplochromines – including Pseudocrenilabrus philander – are poorly supported and differ between the analyses. LM: Lake Malawi, LV: Lake Victoria, LT: Lake Tanganyika Supplementary Figure 2 | Synteny analysis of teleost fhl2 paralogs. Dotplots of the human FHL2 gene region on human chr2 (100-220Mb) shows double conserved synteny to the two fhl2 paralogons in (a) medaka on chromosomes Ola21 (fhl2a) and Ola2 (fhl2b) and in (b) zebrafish on chromosomes Dre9 (fhl2b) and Dre6 (fhl2b). These chromosomes were previously shown to be derived from the ancestral chromosome c and duplicated during the teleost genome duplication1,2. Supplementary Figure 3 | Gene expression profiling in the teleost Danio rerio and Oryzias latipes. (a) Relative quantification (RQ) of fhl2a and fhl2b gene expression in ten tissues in D. rerio (three replicates per tissue) (b) RQ of fhl2a and fhl2b gene expression in eleven tissues in O. latipes (three replicates per tissue). In both species, gill tissue was used as reference. The error bars represent the standard error of the mean (SEM). In D. rerio (a) expression of fhl2a is higher in heart, eye, and oral jaw, although the expression of this gene copy is overall very low, especially when compared to the level of fhl2a expression in cichlids and O. latipes. Contrary to the scenario in cichlids (Fig. -

Mating Systems in Cooperative Breeders : the Roles of Resource Dispersion and Conflict Mitigation
Erschienen in: Behavioral Ecology ; 23 (2012), 3. - S. 521-530 https://dx.doi.org/10.1093/beheco/arr218 Mating systems in cooperative breeders: the roles of resource dispersion and conflict mitigation M. Y. L. Wong,a L. A. Jordan,b S. Marsh-Rollo,c S. St-Cyr,d J. O. Reynolds,e K. A. Stiver,f J. K. Desjardins,g J. L. Fitzpatrick,h and S. Balshinec aDepartment of Biology, Boston University, 5 Cummington Street, Boston, MA 02215, USA, bSchool of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia, cDepartment of Psychology, Neuroscience and Behaviour, McMaster University, 1280 Main Street West, Hamilton, Ontario L8S 4K1, Canada, dDepartment of Ecology and Evolutionary Biology, University of Toronto, 25 Willcocks Street, Ontario M5S 3B2, Canada, eVancouver Aquarium, 845 Avison Way, Vancouver, British Columbia V6G 3E2, Canada, fDepartment of Ecology and Evolutionary Biology, Yale University, 165 Prospect Street, New Haven, CT 06520, USA, gDepartment of Biology, Stanford University, Stanford, Gilbert Hall, CA 94305, USA, and hCentre for Evolutionary Biology, University of Western Australia, 35 Stirling Highway, Crawley, Western Australia 6009, Australia Within animal societies, the ecological and social underpinnings of mating system variation can be related to resource dispersion, sexual conflict between breeders, and the effects of non breeders. Here, we conducted a broad scale investigation into the evolution of mating systems in the cooperatively breeding cichlid, Neolamprologus pulcher, a species that exhibits both monogamy and polygyny within populations. Using long term field data, we showed that polygynous groups were more spatially clustered and held by larger competitively superior males than were monogamous groups, supporting the role of resource dispersion. -

Strategic and Tactical Fighting Decisions in Cichlid Fishes With
Behaviour 151 (2014) 47–71 brill.com/beh Strategic and tactical fighting decisions in cichlid fishes with divergent social systems a b b, Kristina Hick , Adam R. Reddon , Constance M. O’Connor ∗ and Sigal Balshine b a Department of Biology, McMaster University, 1280 Main Street West, Hamilton, ON, Canada L8S 4K1 b Department of Psychology, Neuroscience & Behaviour, McMaster University, 1280 Main Street West, Hamilton, ON, Canada L8S 4K1 *Corresponding author’s e-mail address: [email protected] Accepted 9 August 2013; published online 2 October 2013 Abstract The costs and benefits of engaging in a contest will differ depending on the social situation of the individuals involved. Therefore, understanding contest behaviour can help to elucidate the trade- offs of living in differing social systems and shed light on the evolution of social behaviour. In the current study, we compared contest behaviour in two closely related species of Lamprologine cichlid fish. Neolamprologus pulcher and Telmatochromis temporalis are both pair-breeding cich- lids, but N. pulcher are highly social, group-living fish, while T. temporalis display no grouping behaviour. To examine how competition varies by species, sex and familiarity, we staged same-sex conspecific contests over a shelter, a resource that is highly valued by both species, where contes- tants were either familiar or unfamiliar to one another. When we examined tactical and strategic components of these contests, we found that the highly social species had shorter contests and engaged in fewer costly aggressive acts than did the non-social species. Individuals of the highly social species were also more likely to resolve conflicts through the use of submissive displays, while individuals of the non-social species were more likely to flee from conflict.