Massive Windborne Migration of Sahelian Insects
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Diptera: Calyptratae)
Systematic Entomology (2020), DOI: 10.1111/syen.12443 Protein-encoding ultraconserved elements provide a new phylogenomic perspective of Oestroidea flies (Diptera: Calyptratae) ELIANA BUENAVENTURA1,2 , MICHAEL W. LLOYD2,3,JUAN MANUEL PERILLALÓPEZ4, VANESSA L. GONZÁLEZ2, ARIANNA THOMAS-CABIANCA5 andTORSTEN DIKOW2 1Museum für Naturkunde, Leibniz Institute for Evolution and Biodiversity Science, Berlin, Germany, 2National Museum of Natural History, Smithsonian Institution, Washington, DC, U.S.A., 3The Jackson Laboratory, Bar Harbor, ME, U.S.A., 4Department of Biological Sciences, Wright State University, Dayton, OH, U.S.A. and 5Department of Environmental Science and Natural Resources, University of Alicante, Alicante, Spain Abstract. The diverse superfamily Oestroidea with more than 15 000 known species includes among others blow flies, flesh flies, bot flies and the diverse tachinid flies. Oestroidea exhibit strikingly divergent morphological and ecological traits, but even with a variety of data sources and inferences there is no consensus on the relationships among major Oestroidea lineages. Phylogenomic inferences derived from targeted enrichment of ultraconserved elements or UCEs have emerged as a promising method for resolving difficult phylogenetic problems at varying timescales. To reconstruct phylogenetic relationships among families of Oestroidea, we obtained UCE loci exclusively derived from the transcribed portion of the genome, making them suitable for larger and more integrative phylogenomic studies using other genomic and transcriptomic resources. We analysed datasets containing 37–2077 UCE loci from 98 representatives of all oestroid families (except Ulurumyiidae and Mystacinobiidae) and seven calyptrate outgroups, with a total concatenated aligned length between 10 and 550 Mb. About 35% of the sampled taxa consisted of museum specimens (2–92 years old), of which 85% resulted in successful UCE enrichment. -

The Influence of Prairie Restoration on Hemiptera
CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Thesis Submitted to The College of Arts and Sciences of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Biology By Stephanie Kay Gunter, B.A. Dayton, Ohio August 2021 CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay APPROVED BY: Chelse M. Prather, Ph.D. Faculty Advisor Associate Professor Department of Biology Ryan W. McEwan, Ph.D. Committee Member Associate Professor Department of Biology Mark G. Nielsen Ph.D. Committee Member Associate Professor Department of Biology ii © Copyright by Stephanie Kay Gunter All rights reserved 2021 iii ABSTRACT CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay University of Dayton Advisor: Dr. Chelse M. Prather Ohio historically hosted a patchwork of tallgrass prairies, which provided habitat for native species and prevented erosion. As these vulnerable habitats have declined in the last 200 years due to increased human land use, restorations of these ecosystems have increased, and it is important to evaluate their success. The Hemiptera (true bugs) are an abundant and varied order of insects including leafhoppers, aphids, cicadas, stink bugs, and more. They play important roles in grassland ecosystems, feeding on plant sap and providing prey to predators. Hemipteran abundance and composition can respond to grassland restorations, age of restoration, and size and isolation of habitat. -

A New Species of the Genus Euricania Melichar
Zootaxa 4033 (1): 137–143 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.4033.1.8 http://zoobank.org/urn:lsid:zoobank.org:pub:8A95CF0B-BCD9-4D34-AD74-F1C3DA4616D6 A new species of the genus Euricania Melichar, 1898 (Hemiptera: Fulgoromorpha: Ricaniidae) from China, with a world checklist and a key to all species recorded for the country LAN-LAN REN1, ADAM STROIŃSKI2 & DAO-ZHENG QIN1,* 1Key Laboratory of Plant Protection Resources and Pest Management of the Ministry of Education; Entomological Museum, North- west A&F University, Yangling, Shaanxi 712100, China 2Museum and Institute of Zoology, Polish Academy of Sciences, 64, Wilcza Street, 00-679 Warsaw, Poland 3Corresponding author. E-mail: [email protected] Abstract One new species of the planthopper genus Euricania Melichar, 1898 – E. paraclara sp. nov. is described from Guizhou (southwest China). A checklist of all Euricania species and an identification key to the species of the Chinese fauna are provided. Photographs of the adult and illustrations of male and female genitalia of the new species are also given. Key words: Fulgoroidea, planthopper, taxonomy, key, checklist Introduction The planthopper genus Euricania (Hemiptera: Ricaniidae) was established by Melichar (1898a) with the type species Pochazia ocellus Walker, 1851 designated subsequently by Distant (1906). It is a relatively large genus in the family Ricaniidae with 36 species and subspecies (Bourgoin 2015), widely distributed in the southeastern Palaearctic (China, Japan), Oriental Region (India, Bangladesh, Indonesia, Malaysia, Taiwan), New Guinea, Solomon Islands, Vanuatu, Fiji and North Australia (Fletcher 2008). -

Insects & Spiders of Kanha Tiger Reserve
Some Insects & Spiders of Kanha Tiger Reserve Some by Aniruddha Dhamorikar Insects & Spiders of Kanha Tiger Reserve Aniruddha Dhamorikar 1 2 Study of some Insect orders (Insecta) and Spiders (Arachnida: Araneae) of Kanha Tiger Reserve by The Corbett Foundation Project investigator Aniruddha Dhamorikar Expert advisors Kedar Gore Dr Amol Patwardhan Dr Ashish Tiple Declaration This report is submitted in the fulfillment of the project initiated by The Corbett Foundation under the permission received from the PCCF (Wildlife), Madhya Pradesh, Bhopal, communication code क्रम 車क/ तकनीकी-I / 386 dated January 20, 2014. Kanha Office Admin office Village Baherakhar, P.O. Nikkum 81-88, Atlanta, 8th Floor, 209, Dist Balaghat, Nariman Point, Mumbai, Madhya Pradesh 481116 Maharashtra 400021 Tel.: +91 7636290300 Tel.: +91 22 614666400 [email protected] www.corbettfoundation.org 3 Some Insects and Spiders of Kanha Tiger Reserve by Aniruddha Dhamorikar © The Corbett Foundation. 2015. All rights reserved. No part of this book may be used, reproduced, or transmitted in any form (electronic and in print) for commercial purposes. This book is meant for educational purposes only, and can be reproduced or transmitted electronically or in print with due credit to the author and the publisher. All images are © Aniruddha Dhamorikar unless otherwise mentioned. Image credits (used under Creative Commons): Amol Patwardhan: Mottled emigrant (plate 1.l) Dinesh Valke: Whirligig beetle (plate 10.h) Jeffrey W. Lotz: Kerria lacca (plate 14.o) Piotr Naskrecki, Bud bug (plate 17.e) Beatriz Moisset: Sweat bee (plate 26.h) Lindsay Condon: Mole cricket (plate 28.l) Ashish Tiple: Common hooktail (plate 29.d) Ashish Tiple: Common clubtail (plate 29.e) Aleksandr: Lacewing larva (plate 34.c) Jeff Holman: Flea (plate 35.j) Kosta Mumcuoglu: Louse (plate 35.m) Erturac: Flea (plate 35.n) Cover: Amyciaea forticeps preying on Oecophylla smargdina, with a kleptoparasitic Phorid fly sharing in the meal. -
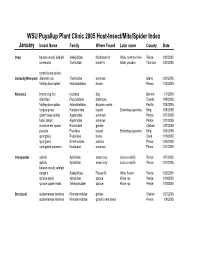
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Large Positive Ecological Changes of Small Urban Greening Actions Luis Mata, Amy K. Hahs, Estibaliz Palma, Anna Backstrom, Tyler King, Ashley R
Large positive ecological changes of small urban greening actions Luis Mata, Amy K. Hahs, Estibaliz Palma, Anna Backstrom, Tyler King, Ashley R. Olson, Christina Renowden, Tessa R. Smith and Blythe Vogel WebPanel 2 Table S2.1. List of the 94 insect species that were recorded during the study. DET: Detritivore; HER: Herbivore; PRE: Predator; PAR: Parasitoid. All species are indigenous to the study area, excepting those marked with an *. Year 0 Year 1 Year 2 Year 3 Species/morphospecies Common name Family DET HER PRE PAR [2016] [2017] [2018] [2019] Hymenoptera | Apocrita Apocrita 5 Apocrita 5 Apocrita 33 Apocrita 33 Apocrita 36 Apocrita 36 Apocrita 37 Apocrita 37 Apocrita 40 Apocrita 40 Apocrita 44 Apocrita 44 Apocrita 46 Apocrita 46 Apocrita 48 Apocrita 48 Apocrita 49 Apocrita 49 Apocrita 51 Apocrita 51 Apocrita 52 Apocrita 52 Apocrita 53 Apocrita 53 Apocrita 54 Apocrita 54 Apocrita 55 Apocrita 55 Apocrita 56 Apocrita 56 Apocrita 57 Apocrita 57 Apocrita 58 Apocrita 58 Apocrita 59 Apocrita 59 Apocrita 60 Apocrita 60 Apocrita 61 Apocrita 61 Apocrita 62 Apocrita 62 Apocrita 65 Apocrita 65 Apocrita 74 Apocrita 74 Apocrita 89 Apocrita 89 Apocrita 90 Apocrita 90 Apocrita 91 Apocrita 91 Hymenoptera | Apoidea | Anthophila Anthophila 1 Anthophila 1 Anthophila 3 Anthophila 3 Apis mellifera* European honeybee Apidae Diptera | Brachycera Brachycera 2 Brachycera 2 Brachycera 7 Brachycera 7 Brachycera 8 Brachycera 8 Brachycera 14 Brachycera 14 Brachycera 15 Brachycera 15 Brachycera 16 Brachycera 16 Brachycera 18 Brachycera 18 Brachycera 19 Brachycera -

A Comparison of the External Morphology and Functions of Labial Tip Sensilla in Semiaquatic Bugs (Hemiptera: Heteroptera: Gerromorpha)
Eur. J. Entomol. 111(2): 275–297, 2014 doi: 10.14411/eje.2014.033 ISSN 1210-5759 (print), 1802-8829 (online) A comparison of the external morphology and functions of labial tip sensilla in semiaquatic bugs (Hemiptera: Heteroptera: Gerromorpha) 1 2 JOLANTA BROŻeK and HERBERT ZeTTeL 1 Department of Zoology, Faculty of Biology and environmental Protection, University of Silesia, Bankowa 9, PL 40-007 Katowice, Poland; e-mail: [email protected] 2 Natural History Museum, entomological Department, Burgring 7, 1010 Vienna, Austria; e-mail: [email protected] Key words. Heteroptera, Gerromorpha, labial tip sensilla, pattern, morphology, function, apomorphic characters Abstract. The present study provides new data on the morphology and distribution of the labial tip sensilla of 41 species of 20 gerro- morphan (sub)families (Heteroptera: Gerromorpha) obtained using a scanning electron microscope. There are eleven morphologically distinct types of sensilla on the tip of the labium: four types of basiconic uniporous sensilla, two types of plate sensilla, one type of peg uniporous sensilla, peg-in-pit sensilla, dome-shaped sensilla, placoid multiporous sensilla and elongated placoid multiporous sub- apical sensilla. Based on their external structure, it is likely that these sensilla are thermo-hygrosensitive, chemosensitive and mechano- chemosensitive. There are three different designs of sensilla in the Gerromorpha: the basic design occurs in Mesoveliidae and Hebridae; the intermediate one is typical of Hydrometridae and Hermatobatidae, and the most specialized design in Macroveliidae, Veliidae and Gerridae. No new synapomorphies for Gerromorpha were identified in terms of the labial tip sensilla, multi-peg structures and shape of the labial tip, but eleven new diagnostic characters are recorded for clades currently recognized in this infraorder. -

Nomenclatural Studies Toward a World List of Diptera Genus-Group Names
Nomenclatural studies toward a world list of Diptera genus-group names. Part V Pierre-Justin-Marie Macquart Evenhuis, Neal L.; Pape, Thomas; Pont, Adrian C. DOI: 10.11646/zootaxa.4172.1.1 Publication date: 2016 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Evenhuis, N. L., Pape, T., & Pont, A. C. (2016). Nomenclatural studies toward a world list of Diptera genus- group names. Part V: Pierre-Justin-Marie Macquart. Magnolia Press. Zootaxa Vol. 4172 No. 1 https://doi.org/10.11646/zootaxa.4172.1.1 Download date: 02. Oct. 2021 Zootaxa 4172 (1): 001–211 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4172.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:22128906-32FA-4A80-85D6-10F114E81A7B ZOOTAXA 4172 Nomenclatural Studies Toward a World List of Diptera Genus-Group Names. Part V: Pierre-Justin-Marie Macquart NEAL L. EVENHUIS1, THOMAS PAPE2 & ADRIAN C. PONT3 1 J. Linsley Gressitt Center for Entomological Research, Bishop Museum, 1525 Bernice Street, Honolulu, Hawaii 96817-2704, USA. E-mail: [email protected] 2 Natural History Museum of Denmark, Universitetsparken 15, 2100 Copenhagen, Denmark. E-mail: [email protected] 3Oxford University Museum of Natural History, Parks Road, Oxford OX1 3PW, UK. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by D. Whitmore: 15 Aug. 2016; published: 30 Sept. 2016 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 NEAL L. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

Species Distribution Ranking for the Multiple Projects Project Organism Group: Hemiptera Specimens
Species Distribution Ranking for the Multiple Projects Project Organism Group: Hemiptera Specimens Family and Species Sum Of Sites Where Species Was Found Cercopidae Clastoptera obtusa (Spittlebug) 26 Cercopidae Prosapia bicincta (Two-lined spittle bug) 24 Delphacidae Liburniella ornata (Planthopper) 21 Cicadellidae Jikradia olitorius (Leafhopper) 18 Miridae Lygus lineolaris (Tarnished plant bug) 18 Cercopidae Philaenus spumarius (Meadow spittlebug) 18 Berytidae Jalysus spinosus (Stilt bug) 18 Cercopidae Clastoptera xanthocephala (Spittlebug) 16 Cicadellidae Graphocephala coccinea (Leafhopper) 15 Pentatomidae Mormidea lugens (Stink bug) 12 Alydidae Alydus eurinus (Broad-headed bug) 12 Cercopidae Lepyronia quadrangularis (Spittlebug) 11 Pentatomidae Euschistus tristigmus (Stink bug) 11 Rhyparochromidae Pseudopachybrachius basalis (Seed bug) 10 Lygaeidae Kleidocerys resedae (Seed bug) 10 Psyllidae Cacopsylla carpinicola (Psyllid plant bug) 9 Rhopalidae Niesthrea louisianica (Scentless plant bug) 9 Cydnidae Sehirus cinctus (Burrower bug) 9 Cercopidae Aphrophora saratogenesis (Spittlebug) 9 Flatidae Metcalfa pruinosa (Planthopper) 9 Flatidae Anormenis chloris (Planthopper) 9 Psyllidae Bactericera tripunctata (Jumping plant lice) 8 Delphacidae Isodelphax basivitta (Planthopper) 8 Delphacidae Delphacodes puella (Planthopper) 8 Psyllidae Bactericera species (Jumping plant lice) 8 Cercopidae Aphrophora quadrinotata (Spittlebug) 8 Cercopidae Aphrophora cribrata (Pine spittle bug) 7 Pentatomidae Euschistus servus (Stink bug) 7 Membracidae Acutalis -

The Insect Database in Dokdo, Korea: an Updated Version in 2020
Biodiversity Data Journal 9: e62011 doi: 10.3897/BDJ.9.e62011 Data Paper The Insect database in Dokdo, Korea: An updated version in 2020 Jihun Ryu‡,§, Young-Kun Kim |, Sang Jae Suh|, Kwang Shik Choi‡,§,¶ ‡ School of Life Science, BK21 FOUR KNU Creative BioResearch Group, Kyungpook National University, Daegu, South Korea § Research Institute for Dok-do and Ulleung-do Island, Kyungpook National University, Daegu, South Korea | School of Applied Biosciences, Kyungpook National University, Daegu, South Korea ¶ Research Institute for Phylogenomics and Evolution, Kyungpook National University, Daegu, South Korea Corresponding author: Kwang Shik Choi ([email protected]) Academic editor: Paulo Borges Received: 14 Dec 2020 | Accepted: 20 Jan 2021 | Published: 26 Jan 2021 Citation: Ryu J, Kim Y-K, Suh SJ, Choi KS (2021) The Insect database in Dokdo, Korea: An updated version in 2020. Biodiversity Data Journal 9: e62011. https://doi.org/10.3897/BDJ.9.e62011 Abstract Background Dokdo, a group of islands near the East Coast of South Korea, comprises 89 small islands. These volcanic islands were created by an eruption that also led to the formation of the Ulleungdo Islands (located in the East Sea), which are approximately 87.525 km away from Dokdo. Dokdo is important for geopolitical reasons; however, because of certain barriers to investigation, such as weather and time constraints, knowledge of its insect fauna is limited compared to that of Ulleungdo. Until 2017, insect fauna on Dokdo included 10 orders, 74 families, 165 species and 23 undetermined species; subsequently, from 2018 to 2019, we discovered 23 previously unrecorded species and three undetermined species via an insect survey. -

Building-Up of a DNA Barcode Library for True Bugs (Insecta: Hemiptera: Heteroptera) of Germany Reveals Taxonomic Uncertainties and Surprises
Building-Up of a DNA Barcode Library for True Bugs (Insecta: Hemiptera: Heteroptera) of Germany Reveals Taxonomic Uncertainties and Surprises Michael J. Raupach1*, Lars Hendrich2*, Stefan M. Ku¨ chler3, Fabian Deister1,Je´rome Morinie`re4, Martin M. Gossner5 1 Molecular Taxonomy of Marine Organisms, German Center of Marine Biodiversity (DZMB), Senckenberg am Meer, Wilhelmshaven, Germany, 2 Sektion Insecta varia, Bavarian State Collection of Zoology (SNSB – ZSM), Mu¨nchen, Germany, 3 Department of Animal Ecology II, University of Bayreuth, Bayreuth, Germany, 4 Taxonomic coordinator – Barcoding Fauna Bavarica, Bavarian State Collection of Zoology (SNSB – ZSM), Mu¨nchen, Germany, 5 Terrestrial Ecology Research Group, Department of Ecology and Ecosystem Management, Technische Universita¨tMu¨nchen, Freising-Weihenstephan, Germany Abstract During the last few years, DNA barcoding has become an efficient method for the identification of species. In the case of insects, most published DNA barcoding studies focus on species of the Ephemeroptera, Trichoptera, Hymenoptera and especially Lepidoptera. In this study we test the efficiency of DNA barcoding for true bugs (Hemiptera: Heteroptera), an ecological and economical highly important as well as morphologically diverse insect taxon. As part of our study we analyzed DNA barcodes for 1742 specimens of 457 species, comprising 39 families of the Heteroptera. We found low nucleotide distances with a minimum pairwise K2P distance ,2.2% within 21 species pairs (39 species). For ten of these species pairs (18 species), minimum pairwise distances were zero. In contrast to this, deep intraspecific sequence divergences with maximum pairwise distances .2.2% were detected for 16 traditionally recognized and valid species. With a successful identification rate of 91.5% (418 species) our study emphasizes the use of DNA barcodes for the identification of true bugs and represents an important step in building-up a comprehensive barcode library for true bugs in Germany and Central Europe as well.