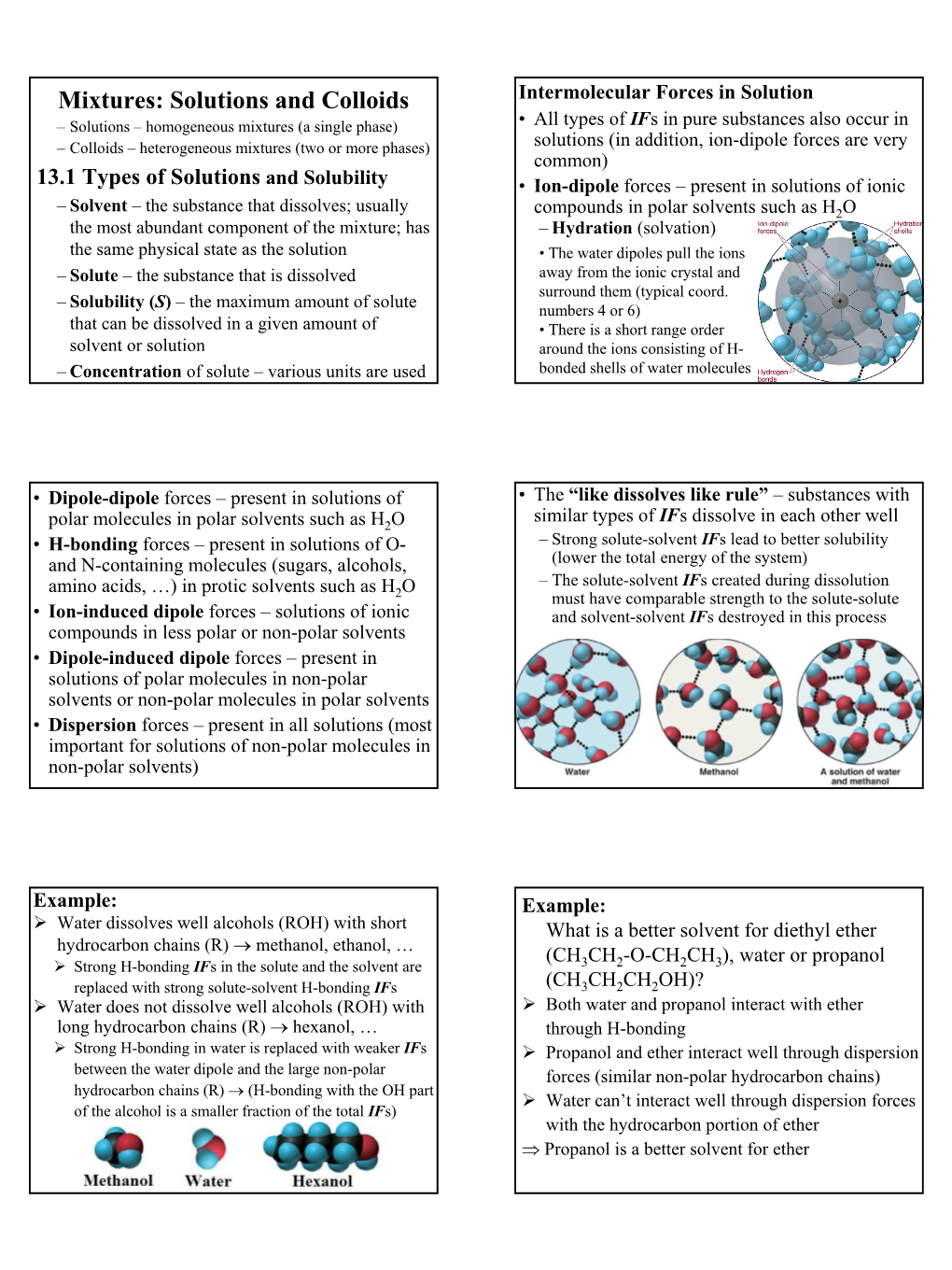
Mixtures: Solutions and Colloids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Page 1 of 6 This Is Henry's Law. It Says That at Equilibrium the Ratio of Dissolved NH3 to the Partial Pressure of NH3 Gas In
CHMY 361 HANDOUT#6 October 28, 2012 HOMEWORK #4 Key Was due Friday, Oct. 26 1. Using only data from Table A5, what is the boiling point of water deep in a mine that is so far below sea level that the atmospheric pressure is 1.17 atm? 0 ΔH vap = +44.02 kJ/mol H20(l) --> H2O(g) Q= PH2O /XH2O = K, at ⎛ P2 ⎞ ⎛ K 2 ⎞ ΔH vap ⎛ 1 1 ⎞ ln⎜ ⎟ = ln⎜ ⎟ − ⎜ − ⎟ equilibrium, i.e., the Vapor Pressure ⎝ P1 ⎠ ⎝ K1 ⎠ R ⎝ T2 T1 ⎠ for the pure liquid. ⎛1.17 ⎞ 44,020 ⎛ 1 1 ⎞ ln⎜ ⎟ = − ⎜ − ⎟ = 1 8.3145 ⎜ T 373 ⎟ ⎝ ⎠ ⎝ 2 ⎠ ⎡1.17⎤ − 8.3145ln 1 ⎢ 1 ⎥ 1 = ⎣ ⎦ + = .002651 T2 44,020 373 T2 = 377 2. From table A5, calculate the Henry’s Law constant (i.e., equilibrium constant) for dissolving of NH3(g) in water at 298 K and 340 K. It should have units of Matm-1;What would it be in atm per mole fraction, as in Table 5.1 at 298 K? o For NH3(g) ----> NH3(aq) ΔG = -26.5 - (-16.45) = -10.05 kJ/mol ΔG0 − [NH (aq)] K = e RT = 0.0173 = 3 This is Henry’s Law. It says that at equilibrium the ratio of dissolved P NH3 NH3 to the partial pressure of NH3 gas in contact with the liquid is a constant = 0.0173 (Henry’s Law Constant). This also says [NH3(aq)] =0.0173PNH3 or -1 PNH3 = 0.0173 [NH3(aq)] = 57.8 atm/M x [NH3(aq)] The latter form is like Table 5.1 except it has NH3 concentration in M instead of XNH3. -

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

Introduction to the Solubility of Liquids in Liquids
INTRODUCTION TO THE SOLUBILITY OF LIQUIDS IN LIQUIDS The Solubility Data Series is made up of volumes of comprehensive and critically evaluated solubility data on chemical systems in clearly defined areas. Data of suitable precision are presented on data sheets in a uniform format, preceded for each system by a critical evaluation if more than one set of data is available. In those systems where data from different sources agree sufficiently, recommended values are pro posed. In other cases, values may be described as "tentative", "doubtful" or "rejected". This volume is primarily concerned with liquid-liquid systems, but related gas-liquid and solid-liquid systems are included when it is logical and convenient to do so. Solubilities at elevated and low 'temperatures and at elevated pressures may be included, as it is considered inappropriate to establish artificial limits on the data presented. For some systems the two components are miscible in all proportions at certain temperatures or pressures, and data on miscibility gap regions and upper and lower critical solution temperatures are included where appropriate and if available. TERMINOLOGY In this volume a mixture (1,2) or a solution (1,2) refers to a single liquid phase containing components 1 and 2, with no distinction being made between solvent and solute. The solubility of a substance 1 is the relative proportion of 1 in a mixture which is saturated with respect to component 1 at a specified temperature and pressure. (The term "saturated" implies the existence of equilibrium with respect to the processes of mass transfer between phases) • QUANTITIES USED AS MEASURES OF SOLUBILITY Mole fraction of component 1, Xl or x(l): ml/Ml nl/~ni = r(m.IM.) '/. -

Chapter 15: Solutions
452-487_Ch15-866418 5/10/06 10:51 AM Page 452 CHAPTER 15 Solutions Chemistry 6.b, 6.c, 6.d, 6.e, 7.b I&E 1.a, 1.b, 1.c, 1.d, 1.j, 1.m What You’ll Learn ▲ You will describe and cate- gorize solutions. ▲ You will calculate concen- trations of solutions. ▲ You will analyze the colliga- tive properties of solutions. ▲ You will compare and con- trast heterogeneous mixtures. Why It’s Important The air you breathe, the fluids in your body, and some of the foods you ingest are solu- tions. Because solutions are so common, learning about their behavior is fundamental to understanding chemistry. Visit the Chemistry Web site at chemistrymc.com to find links about solutions. Though it isn’t apparent, there are at least three different solu- tions in this photo; the air, the lake in the foreground, and the steel used in the construction of the buildings are all solutions. 452 Chapter 15 452-487_Ch15-866418 5/10/06 10:52 AM Page 453 DISCOVERY LAB Solution Formation Chemistry 6.b, 7.b I&E 1.d he intermolecular forces among dissolving particles and the Tattractive forces between solute and solvent particles result in an overall energy change. Can this change be observed? Safety Precautions Dispose of solutions by flushing them down a drain with excess water. Procedure 1. Measure 10 g of ammonium chloride (NH4Cl) and place it in a Materials 100-mL beaker. balance 2. Add 30 mL of water to the NH4Cl, stirring with your stirring rod. -

Lecture 3. the Basic Properties of the Natural Atmosphere 1. Composition
Lecture 3. The basic properties of the natural atmosphere Objectives: 1. Composition of air. 2. Pressure. 3. Temperature. 4. Density. 5. Concentration. Mole. Mixing ratio. 6. Gas laws. 7. Dry air and moist air. Readings: Turco: p.11-27, 38-43, 366-367, 490-492; Brimblecombe: p. 1-5 1. Composition of air. The word atmosphere derives from the Greek atmo (vapor) and spherios (sphere). The Earth’s atmosphere is a mixture of gases that we call air. Air usually contains a number of small particles (atmospheric aerosols), clouds of condensed water, and ice cloud. NOTE : The atmosphere is a thin veil of gases; if our planet were the size of an apple, its atmosphere would be thick as the apple peel. Some 80% of the mass of the atmosphere is within 10 km of the surface of the Earth, which has a diameter of about 12,742 km. The Earth’s atmosphere as a mixture of gases is characterized by pressure, temperature, and density which vary with altitude (will be discussed in Lecture 4). The atmosphere below about 100 km is called Homosphere. This part of the atmosphere consists of uniform mixtures of gases as illustrated in Table 3.1. 1 Table 3.1. The composition of air. Gases Fraction of air Constant gases Nitrogen, N2 78.08% Oxygen, O2 20.95% Argon, Ar 0.93% Neon, Ne 0.0018% Helium, He 0.0005% Krypton, Kr 0.00011% Xenon, Xe 0.000009% Variable gases Water vapor, H2O 4.0% (maximum, in the tropics) 0.00001% (minimum, at the South Pole) Carbon dioxide, CO2 0.0365% (increasing ~0.4% per year) Methane, CH4 ~0.00018% (increases due to agriculture) Hydrogen, H2 ~0.00006% Nitrous oxide, N2O ~0.00003% Carbon monoxide, CO ~0.000009% Ozone, O3 ~0.000001% - 0.0004% Fluorocarbon 12, CF2Cl2 ~0.00000005% Other gases 1% Oxygen 21% Nitrogen 78% 2 • Some gases in Table 3.1 are called constant gases because the ratio of the number of molecules for each gas and the total number of molecules of air do not change substantially from time to time or place to place. -

Chemistry C3102-2006: Polymers Section Dr. Edie Sevick, Research School of Chemistry, ANU 5.0 Thermodynamics of Polymer Solution
Chemistry C3102-2006: Polymers Section Dr. Edie Sevick, Research School of Chemistry, ANU 5.0 Thermodynamics of Polymer Solutions In this section, we investigate the solubility of polymers in small molecule solvents. Solubility, whether a chain goes “into solution”, i.e. is dissolved in solvent, is an important property. Full solubility is advantageous in processing of polymers; but it is also important for polymers to be fully insoluble - think of plastic shoe soles on a rainy day! So far, we have briefly touched upon thermodynamic solubility of a single chain- a “good” solvent swells a chain, or mixes with the monomers, while a“poor” solvent “de-mixes” the chain, causing it to collapse upon itself. Whether two components mix to form a homogeneous solution or not is determined by minimisation of a free energy. Here we will express free energy in terms of canonical variables T,P,N , i.e., temperature, pressure, and number (of moles) of molecules. The free energy { } expressed in these variables is the Gibbs free energy G G(T,P,N). (1) ≡ In previous sections, we referred to the Helmholtz free energy, F , the free energy in terms of the variables T,V,N . Let ∆Gm denote the free eneregy change upon homogeneous mix- { } ing. For a 2-component system, i.e. a solute-solvent system, this is simply the difference in the free energies of the solute-solvent mixture and pure quantities of solute and solvent: ∆Gm G(T,P,N , N ) (G0(T,P,N )+ G0(T,P,N )), where the superscript 0 denotes the ≡ 1 2 − 1 2 pure component. -

Gp-Cpc-01 Units – Composition – Basic Ideas
GP-CPC-01 UNITS – BASIC IDEAS – COMPOSITION 11-06-2020 Prof.G.Prabhakar Chem Engg, SVU GP-CPC-01 UNITS – CONVERSION (1) ➢ A two term system is followed. A base unit is chosen and the number of base units that represent the quantity is added ahead of the base unit. Number Base unit Eg : 2 kg, 4 meters , 60 seconds ➢ Manipulations Possible : • If the nature & base unit are the same, direct addition / subtraction is permitted 2 m + 4 m = 6m ; 5 kg – 2.5 kg = 2.5 kg • If the nature is the same but the base unit is different , say, 1 m + 10 c m both m and the cm are length units but do not represent identical quantity, Equivalence considered 2 options are available. 1 m is equivalent to 100 cm So, 100 cm + 10 cm = 110 cm 0.01 m is equivalent to 1 cm 1 m + 10 (0.01) m = 1. 1 m • If the nature of the quantity is different, addition / subtraction is NOT possible. Factors used to check equivalence are known as Conversion Factors. GP-CPC-01 UNITS – CONVERSION (2) • For multiplication / division, there are no such restrictions. They give rise to a set called derived units Even if there is divergence in the nature, multiplication / division can be carried out. Eg : Velocity ( length divided by time ) Mass flow rate (Mass divided by time) Mass Flux ( Mass divided by area (Length 2) – time). Force (Mass * Acceleration = Mass * Length / time 2) In derived units, each unit is to be individually converted to suit the requirement Density = 500 kg / m3 . -

Topic720 Composition: Mole Fraction: Molality: Concentration a Solution Comprises at Least Two Different Chemical Substances
Topic720 Composition: Mole Fraction: Molality: Concentration A solution comprises at least two different chemical substances where at least one substance is in vast molar excess. The term ‘solution’ is used to describe both solids and liquids. Nevertheless the term ‘solution’ in the absence of the word ‘solid’ refers to a liquid. Chemists are particularly expert at identifying the number and chemical formulae of chemical substances present in a given closed system. Here we explore how the chemical composition of a given system is expressed. We consider a simple system prepared using water()l and urea(s) at ambient temperature and pressure. We designate water as chemical substance 1 and urea as chemical substance j, so that the closed system contains an aqueous solution. The amounts of the two substances are given by n1 = = ()wM11 and nj ()wMjj where w1 and wj are masses; M1 and Mj are the molar masses of the two chemical substances. In these terms, n1 and nj are extensive variables. = ⋅ + ⋅ Mass of solution, w n1 M1 n j M j (a) = ⋅ Mass of solvent, w1 n1 M1 (b) = -1 For water, M1 0.018 kg mol . However in reviewing the properties of solutions, chemists prefer intensive composition variables. Mole Fraction The mole fractions of the two substances x1 and xj are given by the following two equations: =+ =+ xnnn111()j xnnnjj()1 j (c) += Here x1 x j 10. In general terms for a system comprising i - chemical substances, the mole fraction of substance k is given by equation (d). ji= = xnkk/ ∑ n j (d) j=1 ji= = Hence ∑ x j 10. -

16.4 Calculations Involving Colligative Properties 16.4
chem_TE_ch16.fm Page 491 Tuesday, April 18, 2006 11:27 AM 16.4 Calculations Involving Colligative Properties 16.4 1 FOCUS Connecting to Your World Cooking instructions for a wide Guide for Reading variety of foods, from dried pasta to packaged beans to frozen fruits to Objectives fresh vegetables, often call for the addition of a small amount of salt to the Key Concepts • What are two ways of 16.4.1 Solve problems related to the cooking water. Most people like the flavor of expressing the concentration food cooked with salt. But adding salt can of a solution? molality and mole fraction of a have another effect on the cooking pro- • How are freezing-point solution cess. Recall that dissolved salt elevates depression and boiling-point elevation related to molality? 16.4.2 Describe how freezing-point the boiling point of water. Suppose you Vocabulary depression and boiling-point added a teaspoon of salt to two liters of molality (m) elevation are related to water. A teaspoon of salt has a mass of mole fraction molality. about 20 g. Would the resulting boiling molal freezing-point depression K point increase be enough to shorten constant ( f) the time required for cooking? In this molal boiling-point elevation Guide to Reading constant (K ) section, you will learn how to calculate the b amount the boiling point of the cooking Reading Strategy Build Vocabulary L2 water would rise. Before you read, make a list of the vocabulary terms above. As you Graphic Organizers Use a chart to read, write the symbols or formu- las that apply to each term and organize the definitions and the math- Molality and Mole Fraction describe the symbols or formulas ematical formulas associated with each using words. -

Alkali Metal Vapor Pressures & Number Densities for Hybrid Spin Exchange Optical Pumping
Alkali Metal Vapor Pressures & Number Densities for Hybrid Spin Exchange Optical Pumping Jaideep Singh, Peter A. M. Dolph, & William A. Tobias University of Virginia Version 1.95 April 23, 2008 Abstract Vapor pressure curves and number density formulas for the alkali metals are listed and compared from the 1995 CRC, Nesmeyanov, and Killian. Formulas to obtain the temperature, the dimer to monomer density ratio, and the pure vapor ratio given an alkali density are derived. Considerations and formulas for making a prescribed hybrid vapor ratio of alkali to Rb at a prescribed alkali density are presented. Contents 1 Vapor Pressure Curves 2 1.1TheClausius-ClapeyronEquation................................. 2 1.2NumberDensityFormulas...................................... 2 1.3Comparisonwithotherstandardformulas............................. 3 1.4AlkaliDimers............................................. 3 2 Creating Hybrid Mixes 11 2.1Predictingthehybridvaporratio.................................. 11 2.2Findingthedesiredmolefraction.................................. 11 2.3GloveboxMethod........................................... 12 2.4ReactionMethod........................................... 14 1 1 Vapor Pressure Curves 1.1 The Clausius-Clapeyron Equation The saturated vapor pressure above a liquid (solid) is described by the Clausius-Clapeyron equation. It is a consequence of the equality between the chemical potentials of the vapor and liquid (solid). The derivation can be found in any undergraduate text on thermodynamics (e.g. Kittel & Kroemer [1]): Δv · ∂P = L · ∂T/T (1) where P is the pressure, T is the temperature, L is the latent heat of vaporization (sublimation) per particle, and Δv is given by: Vv Vl(s) Δv = vv − vl(s) = − (2) Nv Nl(s) where V is the volume occupied by the particles, N is the number of particles, and the subscripts v & l(s) refer to the vapor & liquid (solid) respectively. -

Earth Gram199 and Trace Constituents C
EARTH GRAM199 AND TRACE CONSTITUENTS C. Justus', A. Duvall',K KeUe? I Morgan Research Corp., NASA, ED44hUorgan. Marshall Space Flight Center, AL. 35812, USA NASA Marshall Space Flight Center, ELM, Marshull Space Flight Center, AL 35812, USA ABSTRACT Global Reference Atmospheric Model (GRAM-99) is an engineering-level model of Earth's atmosphere It provides both mean values and perturbations for density, tempeaature, pressure, and winds, as well as monthly- and geographically-varying trace constituent c- 'om. From 0-27 GRAM thermodynamics and winds are based on National Oceanic and Atmospheric Administration Global Upper Air Climatic Atlas (GUACA) climatology. Above 120 km, GRAM is based on the NASA Marsball Engineering Thermosphere (MET) model. In the intervening altitude region, GRAM is based on Middle Atmosphere Program (MAP) climatology that also forms the basis of the 1986 COSPAR Intunational Reference Atmosphere (CIRA). Atmospheric composition is represented in GRAM by concentrations of both major and minor species. Above 120 km, MET provides concentration values for Nz, @. Ar, 0,He, and H. Below 120 km, species represented also include H20, 0% N@, CO, cH4, and COZ. At COSPAR 2002 a comparison was made between GRAM constituents below 120 km and those provided by Naval Research Laboratory (NRL) climatology. No current need to update GRAM constituent climatology in that height range was identified. This report examines GRAM (MET) C0ilstituent.s between 100 and lo00 km altitudes. Discregancies are noted between GRAM (MET) constituent number densities and mass density or molecular weight. Near 110 km altitude, there is up to about 25% discrepancy between MET number density and mass density (with mass density beii valid and number densities requiring adjustment). -

Ap Chemistry Notes 1-1 Mole Fraction
AP CHEMISTRY NOTES 1-1 MOLE FRACTION Mole Fraction (X) – the number of moles of a substance per total moles of a mixture Mole Fraction = ______Moles A________ Moles A + Moles B + . EXAMPLE: Determine the mole fraction of iodine in a mixture of 0.23 moles of I2 mixed with 1.1 moles of carbon tetrachloride (CCl4). EXAMPLE: A canister contains 2.3 g of PCl5, 1.2 g of PCl3, and 0.75 g of Cl2. Determine the mole fraction of each of these gases. AP CHEMISTRY NOTES 1-2 THE CONCENTRATION OF IONS IN SOLUTION REVIEW EXAMPLE: Determine the molarity of a solution that contains 1.22 g of KI dissolved in water to make 250. mL of solution. EXAMPLE: Determine the concentration of each ion in the following solutions: 0.25 M BaCl2 ___________________________________________________ 1.5 M Cr(OH)3 ___________________________________________________ 0.11 M K3PO4 ___________________________________________________ EXAMPLE: 100. mL of 0.50 M K2SO4 is mixed with 75.0 mL of 0.25 M Al(NO3)3. What is the concentration of each ion in the solution? EXAMPLE: 50. mL of 0.125 M FeCl3 is mixed with 25 mL of 0.220 M FeCl3. What is the resulting molarity of the solution? AP CHEMISTRY NOTES 1-3 THE PREPARATION OF SOLUTIONS BY DILUTION To Prepare a Solution by Dilution: M1 V1 = M2 V2 (stock soln) (new soln) EXAMPLE: How would you prepare 100.0 mL of a 0.40 M solution of MgSO4 from a stock solution that is 2.0 M in concentration? EXAMPLE: How much water would you add to 25 mL of 1.00 M NaOH to prepare a 0.75 M solution? AP CHEMISTRY NOTES 1-4 LIMITING REAGENTS REVISITED EXAMPLE: 20.0 grams of propane are reacted with 50.0 grams of oxygen at STP according to the following reaction: C3H8 + 5O2 → 3CO2 + 4H2O Determine the limiting reagent: Determine the mass of carbon dioxide produced: Determine the mass of excess reagent that remains after the reaction.