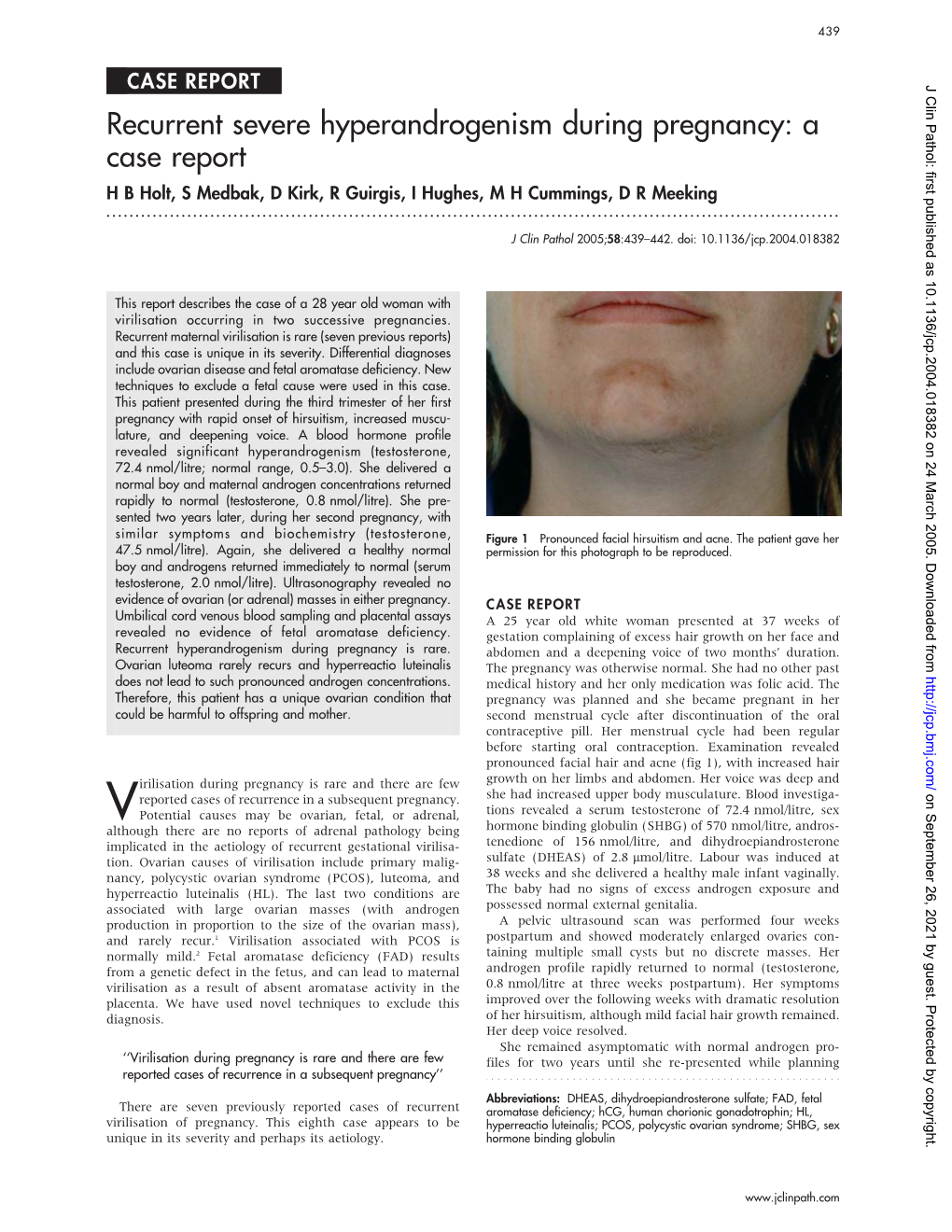
A Case Report H B Holt, S Medbak, D Kirk, R Guirgis, I Hughes, M H Cummings, D R Meeking
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sex Hormones Related Ocular Dryness in Breast Cancer Women
Journal of Clinical Medicine Review Sex Hormones Related Ocular Dryness in Breast Cancer Women Antonella Grasso 1, Antonio Di Zazzo 2,* , Giuseppe Giannaccare 3 , Jaemyoung Sung 4 , Takenori Inomata 4 , Kendrick Co Shih 5 , Alessandra Micera 6, Daniele Gaudenzi 2, Sara Spelta 2 , Maria Angela Romeo 7, Paolo Orsaria 1, Marco Coassin 2 and Vittorio Altomare 1 1 Breast Unit, University Campus Bio-Medico, 00128 Rome, Italy; [email protected] (A.G.); [email protected] (P.O.); [email protected] (V.A.) 2 Ophthalmology Operative Complex Unit, University Campus Bio-Medico, 00128 Rome, Italy; [email protected] (D.G.); [email protected] (S.S.); [email protected] (M.C.) 3 Department of Ophthalmology, University Magna Graecia of Catanzaro, 88100 Catanzaro, Italy; [email protected] 4 Department of Ophthalmology, School of Medicine, Juntendo University, 1130033 Tokyo, Japan; [email protected] (J.S.); [email protected] (T.I.) 5 Department of Ophthalmology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong; [email protected] 6 Research and Development Laboratory for Biochemical, Molecular and Cellular Applications in Ophthalmological Sciences, IRCCS–Fondazione Bietti, 00198 Rome, Italy; [email protected] 7 School of Medicine, Humanitas University, 20089 Milan, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-06225418893; Fax: +39-9622541456 Abstract: Background: Dry eye syndrome (DES) is strictly connected to systemic and topical sex hor- mones. Breast cancer treatment, the subsequent hormonal therapy, the subsequent hyperandrogenism and the early sudden menopause, may be responsible for ocular surface system failure and its clinical Citation: Grasso, A.; Di Zazzo, A.; manifestation as dry eye disease. -

Diverse Pathomechanisms Leading to the Breakdown of Cellular Estrogen Surveillance and Breast Cancer Development: New Therapeutic Strategies
Journal name: Drug Design, Development and Therapy Article Designation: Review Year: 2014 Volume: 8 Drug Design, Development and Therapy Dovepress Running head verso: Suba Running head recto: Diverse pathomechanisms leading to breast cancer development open access to scientific and medical research DOI: http://dx.doi.org/10.2147/DDDT.S70570 Open Access Full Text Article REVIEW Diverse pathomechanisms leading to the breakdown of cellular estrogen surveillance and breast cancer development: new therapeutic strategies Zsuzsanna Suba Abstract: Recognition of the two main pathologic mechanisms equally leading to breast cancer National Institute of Oncology, development may provide explanations for the apparently controversial results obtained by sexual Budapest, Hungary hormone measurements in breast cancer cases. Either insulin resistance or estrogen receptor (ER) defect is the initiator of pathologic processes and both of them may lead to breast cancer development. Primary insulin resistance induces hyperandrogenism and estrogen deficiency, but during these ongoing pathologic processes, ER defect also develops. Conversely, when estrogen resistance is the onset of hormonal and metabolic disturbances, initial counteraction is For personal use only. hyperestrogenism. Compensatory mechanisms improve the damaged reactivity of ERs; however, their failure leads to secondary insulin resistance. The final stage of both pathologic pathways is the breakdown of estrogen surveillance, leading to breast cancer development. Among pre- menopausal breast cancer cases, insulin resistance is the preponderant initiator of alterations with hyperandrogenism, which is reflected by the majority of studies suggesting a causal role of hyperandrogenism in breast cancer development. In the majority of postmenopausal cases, tumor development may also be initiated by insulin resistance, while hyperandrogenism is typi- cally coupled with elevated estrogen levels within the low postmenopausal hormone range. -

139 Normal Bone Density in Male
SEPTEMBER-OCTOBER REV. HOSP. CLÍN. FAC. MED. S. PAULO 56(5):139-142, 2001 NORMAL BONE DENSITY IN MALE PSEUDOHERMAPHRODITISM DUE TO 5α- REDUCTASE 2 DEFICIENCY Elaine Maria Frade Costa, Ivo Jorge Prado Arnhold, Marlene Inacio and Berenice Bilharinho Mendonca RHCFAP/3050 COSTA EMF et al. - Normal bone density in male pseudohermaphroditism due to 5α-reductase 2 deficiency. Rev. Hosp. Clín. Fac. Med. S. Paulo 56(5):139-142, 2001. Bone is an androgen-dependent tissue, but it is not clear whether the androgen action in bone depends on testosterone or on dihydrotestosterone. Patients with 5α-reductase 2 deficiency present normal levels of testosterone and low levels of dihydrotestosterone, providing an in vivo human model for the analysis of the effect of testosterone on bone. Objective: To analyze bone mineral density in 4 adult patients with male pseudohermaphroditism due to 5α-reductase 2 deficiency. Results: Three patients presented normal bone mineral density of the lumbar column (L1-L4) and femur neck, and the other patient presented a slight osteopenia in the lumbar column. Conclusion: Patients with dihydrotestosterone deficiency present normal bone mineral density, suggesting that dihydrotestosterone is not the main androgen acting in bone. DESCRIPTOR: Bone mineral density. Male pseudohermaphroditism. 5α-reductase type 2 deficiency. It has been well documented in the fied androgenic receptors in these cells, fects require aromatization into estro- literature that gonadal steroids regulate thus demonstrating that both androgens gens with subsequent activation of the normal bone metabolism and that in- and estrogens act by a direct mecha- estrogenic receptor. Although it has adequate estrogen concentrations in fe- nism through their respective receptors. -

The Mechanism of Androgen Actions in PCOS Etiology
medical sciences Review The Mechanism of Androgen Actions in PCOS Etiology Valentina Rodriguez Paris 1 and Michael J. Bertoldo 1,2,* 1 Fertility and Research Centre, School of Women’s and Children’s Health, University of New South Wales Sydney, NSW 2052, Australia 2 School of Medical Sciences, University of New South Wales Sydney, NSW 2052, Australia * Correspondence: [email protected] Received: 15 June 2019; Accepted: 20 August 2019; Published: 28 August 2019 Abstract: Polycystic ovary syndrome (PCOS) is the most common endocrine condition in reproductive-age women. By comprising reproductive, endocrine, metabolic and psychological features—the cause of PCOS is still unknown. Consequently, there is no cure, and management is persistently suboptimal as it depends on the ad hoc management of symptoms only. Recently it has been revealed that androgens have an important role in regulating female fertility. Androgen actions are facilitated via the androgen receptor (AR) and transgenic Ar knockout mouse models have established that AR-mediated androgen actions have a part in regulating female fertility and ovarian function. Considerable evidence from human and animal studies currently reinforces the hypothesis that androgens in excess, working via the AR, play a key role in the origins of polycystic ovary syndrome (PCOS). Identifying and confirming the locations of AR-mediated actions and the molecular mechanisms involved in the development of PCOS is critical to provide the knowledge required for the future development of innovative, mechanism-based interventions for the treatment of PCOS. This review summarises fundamental scientific discoveries that have improved our knowledge of androgen actions in PCOS etiology and how this may form the future development of effective methods to reduce symptoms in patients with PCOS. -

CYP19A1 Gene Cytochrome P450 Family 19 Subfamily a Member 1
CYP19A1 gene cytochrome P450 family 19 subfamily A member 1 Normal Function The CYP19A1 gene provides instructions for making an enzyme called aromatase. This enzyme converts a class of hormones called androgens, which are involved in male sexual development, to different forms of the female sex hormone estrogen. In cells, aromatase is found in a structure called the endoplasmic reticulum, which is involved in protein production, processing, and transport. The activity (expression) of aromatase varies among different cell types depending on the cells' need for estrogen. In females, aromatase is most active in the ovaries, where it guides sexual development. In males, aromatase is most active in fat (adipose) tissue. In both males and females, estrogen plays a role in regulating bone growth and blood sugar levels. During fetal development, aromatase converts androgens to estrogens in the placenta, which is the link between the mother's blood supply and the fetus. This conversion in the placenta prevents androgens from directing sexual development in female fetuses. After birth, the conversion of androgens to estrogens takes place in multiple tissues. Health Conditions Related to Genetic Changes Aromatase deficiency More than 20 mutations in the CYP19A1 gene have been found to cause aromatase deficiency. This condition is characterized by reduced levels of estrogen and increased levels of androgens. These abnormal hormone levels lead to impaired sexual development in affected females and unusual bone growth, insulin resistance, and other signs and symptoms in both males and females with the condition. CYP19A1 gene mutations that cause aromatase deficiency decrease or eliminate aromatase activity. A lack of aromatase function results in an inability to convert androgens to estrogens before birth and throughout life. -

Hirsutism and Polycystic Ovary Syndrome (PCOS)
Hirsutism and Polycystic Ovary Syndrome (PCOS) A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction of the Patient Education Committee and the Publications Committee. No portion herein may be reproduced in any form without written permission. This booklet is in no way intended to replace, dictate or fully define evaluation and treatment by a qualified physician. It is intended solely as an aid for patients seeking general information on issues in reproductive medicine. Copyright © 2016 by the American Society for Reproductive Medicine AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE Hirsutism and Polycystic Ovary Syndrome (PCOS) A Guide for Patients Revised 2016 A glossary of italicized words is located at the end of this booklet. INTRODUCTION Hirsutism is the excessive growth of facial or body hair on women. Hirsutism can be seen as coarse, dark hair that may appear on the face, chest, abdomen, back, upper arms, or upper legs. Hirsutism is a symptom of medical disorders associated with the hormones called androgens. Polycystic ovary syndrome (PCOS), in which the ovaries produce excessive amounts of androgens, is the most common cause of hirsutism and may affect up to 10% of women. Hirsutism is very common and often improves with medical management. Prompt medical attention is important because delaying treatment makes the treatment more difficult and may have long-term health consequences. OVERVIEW OF NORMAL HAIR GROWTH Understanding the process of normal hair growth will help you understand hirsutism. Each hair grows from a follicle deep in your skin. As long as these follicles are not completely destroyed, hair will continue to grow even if the shaft, which is the part of the hair that appears above the skin, is plucked or removed. -

A Novel Null Mutation in P450 Aromatase Gene (CYP19A1
J Clin Res Pediatr Endocrinol 2016;8(2):205-210 DO I: 10.4274/jcrpe.2761 Ori gi nal Ar tic le A Novel Null Mutation in P450 Aromatase Gene (CYP19A1) Associated with Development of Hypoplastic Ovaries in Humans Sema Akçurin1, Doğa Türkkahraman2, Woo-Young Kim3, Erdem Durmaz4, Jae-Gook Shin3, Su-Jun Lee3 1Akdeniz University Faculty of Medicine Hospital, Department of Pediatric Endocrinology, Antalya, Turkey 2Antalya Training and Research Hospital, Clinic of Pediatric Endocrinology, Antalya, Turkey 3 Inje University College of Medicine, Department of Pharmacology, Inje University, Busan, Korea 4İzmir University Faculty of Medicine, Medical Park Hospital, Clinic of Pediatric Endocrinology, İzmir, Turkey ABS TRACT Objective: The CYP19A1 gene product aromatase is responsible for estrogen synthesis and androgen/estrogen equilibrium in many tissues, particularly in the placenta and gonads. Aromatase deficiency can cause various clinical phenotypes resulting from excessive androgen accumulation and insufficient estrogen synthesis during the pre- and postnatal periods. In this study, our aim was to determine the clinical characteristics and CYP19A1 mutations in three patients from a large Turkish pedigree. Methods: The cases were the newborns referred to our clinic for clitoromegaly and labial fusion. Virilizing signs such as severe acne formation, voice deepening, and clitoromegaly were noted in the mothers during pregnancy. Preliminary diagnosis was aromatase deficiency. Therefore, direct DNA sequencing of CYP19A1 was performed in samples from parents (n=5) and patients (n=3). WHAT IS ALREADY KNOWN ON THIS TOPIC? Results: In all patients, a novel homozygous insertion mutation in the fifth exon (568insC) was found to cause a frameshift in the open reading frame and to truncate Aromatase deficiency can cause various clinical phenotypes the protein prior to the heme-binding region which is crucial for enzymatic activity. -

Hyperandrogenism by Liquid Chromatography Tandem Mass Spectrometry in PCOS: Focus on Testosterone and Androstenedione
Journal of Clinical Medicine Article Hyperandrogenism by Liquid Chromatography Tandem Mass Spectrometry in PCOS: Focus on Testosterone and Androstenedione Giorgia Grassi 1,* , Elisa Polledri 2, Silvia Fustinoni 2,3 , Iacopo Chiodini 4,5, Ferruccio Ceriotti 6, Simona D’Agostino 6, Francesca Filippi 7, Edgardo Somigliana 2,7, Giovanna Mantovani 1,2, Maura Arosio 1,2 and Valentina Morelli 1 1 Endocrinology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (G.M.); [email protected] (M.A.); [email protected] (V.M.) 2 Department of Clinical Sciences and Community Health, University of Milan, 20122 Milan, Italy; [email protected] (E.P.); [email protected] (S.F.); [email protected] (E.S.) 3 Laboratory of Toxicology, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 4 Department of Medical Biotechnology and Translational Medicine, University of Milan, 20122 Milan, Italy; [email protected] 5 IRCCS Istituto Auxologico, Unit for Bone Metabolism Diseases and Diabetes & Lab of Endocrine and Metabolic Research, Italiano, 20149 Milan, Italy 6 Clinical Laboratory, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (F.C.); [email protected] (S.D.) 7 Infertilty Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; francesca.fi[email protected] * Correspondence: [email protected] Abstract: The identification of hyperandrogenism in polycystic ovary syndrome (PCOS) is concerning Citation: Grassi, G.; Polledri, E.; because of the poor accuracy of the androgen immunoassays (IA) and controversies regarding Fustinoni, S.; Chiodini, I.; Ceriotti, F.; which androgens should be measured. -

A Benign Cause of Hyperandrogenism in a Postmenopausal Woman
ID: 20-0054 -20-0054 J J N Roque and others Hyperandrogenism in ID: 20-0054; February 2021 post-menopause DOI: 10.1530/EDM-20-0054 A benign cause of hyperandrogenism in a postmenopausal woman João José Nunes Roque1, Irina Borisovna Samokhvalova Alves2, Correspondence Ana Maria de Almeida Paiva Fernandes Rodrigues3 and Maria João Bugalho1,4 should be addressed to M J Bugalho 1Department of Endocrinology, Hospital de Santa Maria, Lisboa, Portugal, 2Department of Pathology, Email Hospital de Santa Maria, Lisboa, Portugal, 3Department of Obstetrics & Gynecology, Hospital de Santa Maria, Lisboa, maria.bugalho@chln. Portugal, and 4Faculdade de Medicina da Universidade de Lisboa, Lisboa, Portugal min-saude.pt Summary Menopause is a relative hyperandrogenic state but the development of hirsutism or virilizing features should not be regarded as normal. We report the case of a 62-year-old woman with a 9-month history of progressive frontotemporal hair loss and hirsutism, particularly on her back, arms and forearms. Blood tests showed increased total testosterone of 5.20 nmol/L that remained elevated after an overnight dexamethasone suppression test. Free Androgen Index was 13.1 and DHEAS was repeatedly normal. Imaging examinations to study adrenals and ovaries were negative. The biochemical profileandtheabsenceofimaginginfavorofanadrenaltumormadeusconsidertheovarianoriginasthemostlikely hypothesis. After informed consent, bilateral salpingectomy-oophorectomy and total hysterectomy were performed. Gross pathology revealed ovaries of increased volume -

Spironolactone for Adult Female Acne
® PPracticalEDIATRIC DERMATOLOGY Pearls From the Cutis Board Spironolactone for Adult Female Acne Many cases of acne are hormonal in nature, meaning that they occur in adolescent girls and women and are aggravated by hormonal fluctuations such as those that occur during the menstrual cycle or in the setting of underlying hormonal imbalances as seen in polycystic ovary syndrome. For these patients, antihormonal therapy such as spironolactone is a valid and efficacious option. Herein, initiation and utilization of this medication is reviewed. Adam J. Friedman, MD copy What should you do during the first Evaluation of these women with acne for the visit for a patient you may start possibility of hormonal imbalance may be necessary, on spironolactone? with the 2 most common causes of hyperandrogen- Some women will come in asking about spironolac- ismnot being polycystic ovary syndrome and congeni- tone for acne, so it is important to identify potential tal adrenal hyperplasia. The presence of alopecia, candidates for antihormonal therapy: hirsutism, acanthosis nigricans, or other signs of • Women with acne flares that cycle androgen excess, in combination with dysmenor- with menstruation Dorhea or amenorrhea, may be an indication that the • Women with adult-onset acne or persistent- patient has an underlying medical condition that recurrent acne past teenaged years, even needs to be addressed. Blood tests including testos- in the absence of clinical or laboratory signs terone, dehydroepiandrosterone, follicle-stimulating of hyperandrogenism hormone, and luteinizing hormone would be appro- • Women on oral contraceptives (OCs) who priate screening tests and should be performed dur- exhibit moderate to severe acne, especially ing the menstrual period or week prior; the patient with a hormonal patternCUTIS clinically should not be on an OC or have been on one within • Women not responding to conventional ther- the last 6 weeks of testing. -

October 15, 2015 Special Rapporteur on the Right to Health Office of the United Nations High Commissioner for Human Rights Unit
Katrina Karkazis, PhD, MPH Stanford Center for Biomedical Ethics 1215 Welch Road, Modular A Stanford, CA 94305 telephone: 650-723-5760 fax: 650-725-6131 October 15, 2015 Special Rapporteur on the right to health Office of the United Nations High Commissioner for Human Rights United Nations Office at Geneva, CH-1211 Geneva 10, Switzerland Dear Special Rapporteur, Attached please find my comments on the “Public consultation on sport and healthy lifestyles and the right to health.” I have primarily responded to Question 2. My comments are grounded in my 18-year experience conducting empirical research on controversies over medical care for people born with intersex traits as well as my more recent research on international sports policies that restrict the eligibility of intersex women, which has been a central focus of my research and advocacy over the last 4 years. I have spoken out against the regulations about which I write on a number of occasions and have authored a number of articles and papers on this subject. Most recently, I served as an expert witness at a 2015 challenge to these policies heard at the Court of Arbitration for Sport (CAS) in Lausanne, Switzerland—an appeal which suspended one of these policies. A selection of my written work in this area includes the following, which I have also attached: Karkazis, K., Jordan-Young, R.M., Davis, G., and S. Camporesi. "Out of Bounds? A Critique of Policies on Hyperandrogenism in Elite Female Athletes." The American Journal of Bioethics 12(7): 3-16. Published online June 14, 2012. Karkazis, K., and Jordan-Young, R.M. -

HORMONES and SPORT the Effects of Intense Exercise on the Female
3 HORMONES AND SPORT The effects of intense exercise on the female reproductive system M P Warren and N E Perlroth Department of Obstetrics and Gynecology, Columbia College of Physicians and Surgeons, New York, New York, USA (Requests for offprints should be addressed to M P Warren, Department of Obstetrics and Gynecology, PH 16–20, Columbia University, 622 West 168th Street, New York, New York 10032, USA) Abstract Women have become increasingly physically active in of GnRH include infertility and compromised bone recent decades. While exercise provides substantial health density. Failure to attain peak bone mass and bone loss benefits, intensive exercise is also associated with a unique predispose hypoestrogenic athletes to osteopenia and set of risks for the female athlete. Hypothalamic dysfunc- osteoporosis. tion associated with strenuous exercise, and the resulting Metabolic aberrations associated with nutritional insult disturbance of GnRH pulsatility, can result in delayed may be the primary factors effecting low bone density in menarche and disruption of menstrual cyclicity. hypoestrogenic athletes, thus diagnosis should include Specific mechanisms triggering reproductive dysfunc- careful screening for abnormal eating behavior. Increasing tion may vary across athletic disciplines. An energy drain caloric intake to offset high energy demand may be incurred by women whose energy expenditure exceeds sufficient to reverse menstrual dysfunction and stimulate dietary energy intake appears to be the primary factor bone accretion. Treatment with exogenous estrogen may effecting GnRH suppression in athletes engaged in sports help to curb further bone loss in the hypoestrogenic emphasizing leanness; nutritional restriction may be an amenorrheic athlete, but may not be sufficient to stimulate important causal factor in the hypoestrogenism observed in bone growth.