Spectrochemical Analysis of Solid Samples by Laser-Induced Plasma Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Vacuum Ultra Violet Monochromator I Grating & OEM
Feature Article JY Division nformation Vacuum Ultra Violet Monochromator I Grating & OEM Erick Jourdain Abstract Taking the advantage of Jobin Yvon(JY) leading position in the design and realisation of diffraction grating JY has developed over the last past 30 years some of the most innovative Vacuum Ultra Violet (VUV) monochromators for synchrotron centre. These monochromators, which combine the ultimate advanced diffraction grating technologies and some ultra precise under vacuum mechanics, have been installed in several synchrotron centres and have demonstrated superior performances. Today the development of new compact VUV sources and applications require compact VUV monochromators. Based on diffraction grating capabilities and our large experience acquired in the difficult and demanding market of under vacuum synchrotron monochromators JY is currently developing new compact monochromators for application such as Extreme UV lithography or X-ray Photoemission Spectroscopy. Introduction In the middle of the seventies thank to the development of the aberration corrected holographic toroidal gratings at Jobin Yvon (JY) we introduced on the market a new VUV monochromator concept that drastically enhanced the spectroscopic performances in this field [1]. During the eighties the Toroidal Grating Monochromator (TGM) and its associated toroidal gratings had a large and world wide success. At that time most of the synchrotron VUV beamline got equipped with this monochromator type and even today some recent beamline are still been developed Fig. -

FT-IR Spectroscopy
ORIEL ORIEL PRODUCT TRAINING FT-IR Spectroscopy SECTION FOUR FEATURES • Glossary of Terms • Introduction to FT-IR Spectroscopy Stratford, CT • Toll Free 800.714.5393 Fax 203.378.2457 • www.newport.com/oriel • [email protected] GLOSSARY OF TERMS Before we start the technical discussion on FT-IR Spectrometers, we devote a couple of pages to a Glossary, to facilitate the discussion that follows. 100% Line: Calculated by ratioing two background spectra Collimation: The ideal input beam is a cylinder of light. No taken under identical conditions. Ideally, the result is a flat beam of finite dimensions can be perfectly collimated; at best line at 100% transmittance. there is a diffraction limit. In practice the input beam is a Absorbance: Units used to measure the amount of IR cone that is determined by the source size or aperture used. radiation absorbed by a sample. Absorbance is commonly The degree of collimation can affect the S/N and the used as the Y axis units in IR spectra. Absorbance is defined resolution. by Beer’s Law, and is linearly proportional to concentration. Constructive Interference: A phenomenon that occurs when Aliasing: If frequencies above the Nyquist Frequency are not two waves occupy the same space and are in phase with filtered out, energy in these will appear as spectral artefacts each other. Since the amplitudes of waves are additive, the below the Nyquist Frequency. Optical and electronic anti two waves will add together to give a resultant wave which is aliasing can be used to prevent this. Sometimes the higher more intense than either of the individual waves. -

1. What Is the Difference Between Slit and Monochromator? a Slit Is A
1. What is the difference between slit and monochromator? A slit is a mechanical opening in a black painted metallic sheet, with a circular or triangular hole through which incident rays pass which lead to a monochromator. The slit permits passage of only a small fraction of incident radiation, which is selected for further processing such as wavelength selection and detection etc. Usually the output of the radiation is in the image of the slit. When the slit is wide relative to the wavelength, diffraction is difficult to observe. But when the wavelength and slit opening are of the same order of magnitude, diffraction is observed easily. Here the slit behaves as if it is a source of radiation. The output in this case occurs in a series of 1800 arcs. The plot of the frequency of the radiation as a function of peak transmittance is in the form of a Gaussian curve with maximum intensity being the midpoint of the frequency range. In contrast, monochromator may be defined as a device to select different wavelength of the original source radiation. Usually monochromators used in a UV-Visible/Fluorescence/AAS refer to the combination of slits, lenses, mirrors, windows, prisms and gratings. The output of monochromator is also a narrow band of continuous radiation. The band width is an inverse measure of the quality of the monochromator device. The band widths obtained from filters, prisms, gratings etc., become progressively narrower in each case. 2. Where is the monochromator being placed between source and sample or sample and detector in UV visible spectrophotometer? In a UV- Visible spectrophotometer, the monochromator is placed between the source of light and the sample. -

UV-Vis Product Lineup
Spectrophotometer Series Measurement Bandwidth Wavelength Model Appearance Detector Monochromator Features (functions) wavelength range (nm) (nm) Accuracy Comprehensive Measurement Functions in a Compact Body Transfer Data vis USB Flash Drive to a PC UV-1280 UV-VIS Analyses Covered Using a Single Unit Graphic LCD • An easy-to-see LCD and buttons enables simplified measurement and instrument validation operations. 190 to 1100 5 Silicon photo diode • Equipped with a full range of programs for ultraviolet-visible spectroscopic analysis such as photometric mea- (Standalone or Optional surement, DNA/protein quantitation, and advanced multi-component quantitation. PC Control) • Accommodates a variety of applications utilizing a wealth of accessories compatible with other instruments in the Shimadzu UV series. Aberration-corrected • Save data directly from the unit to a USB flash drive. Data can be displayed using commercially available concave blazed holo- ±1 nm spreadsheet software. • Combined monitor double-beam system for the D WI lamps enables highly stable analyses in a compact unit. BioSpec-nano graphic grating 2 Spectrophotometer for quantitative analysis of nucleic acids and proteins that is especially easy to use For Quantitation of • Measures trace samples as small 1µL (0.2mm optical path length) or 2µL (0.7mm optical path length) Nucleic Acids and 220 to 800 3 Photo diode array • Analytical results can be obtained by simply placing a drop of sample on the measurement window with Proteins a pipette and pressing the measurement button in the software window. Setting the optical path length, (PC Control) measuring, and wiping off the sample is all performed automatically. This eliminates the trouble of raising or lowering the fiber and wiping liquid contact parts with a cloth. -

UV-Vis Spectrophotometry: Monochromators Vs Photodiode Arrays
Technical Note UV-Vis spectrophotometry: monochromators vs photodiode arrays Comparing absorbance measurements between the Quad4 Monochromators™-based Infinite® M200 PRO and a multimode reader using photodiode array technology Introduction Materials and methods The most widely established technology for UV-Vis Infinite M200 PRO multimode microplate reader absorbance measurement is a monochromator-based Multimode microplate reader with a PDA microplate reader. Spectrophotometers have undergone a Herring sperm DNA standard great deal of development since their introduction in the Tris-EDTA early 1950s (1) and, in recent years, multimode readers using Orange G (OG) linear photodiode array (PDA) technology for absorbance ddH2O measurements have become available. PDA-based readers 96-well, transparent UV-Star® plates incorporate an optical grating and a solid state array detector, enabling measurement of light intensity throughout the UV and Experiment 1 visible regions of the spectrum. Similar to a monochromator, Linearity in the visible spectrum using Orange G but much faster, they allow the entire UV-Vis spectrum of a In the first experiment, the OD (optical density) linearity of the sample to be captured within a few seconds per well. PDA spectrophotometer in the visible wavelength range was However, this technology suffers from a number of drawbacks, compared with the OD linearity of the monochromator-based mainly due to high levels of stray light. This results in a Infinite M200 PRO. An OG dilution series was prepared in dramatically limited dynamic measurement range (2). ddH20 (200, 150, 112.5, 84.4, 63.3, 47.5 and 35.6 mg/ml) and This technical note compares the results of basic absorbance 200 µl of each concentration was pipetted into a 96-well measurements performed on an Infinite M200 PRO multimode UV-Star plate in triplicate. -

A Double Beam Spectrometer
A Double Beam Spectrometer A QuickTime movie and a (soundless) GIF animation are both available that illustrate the workings of a double beam spectrophotometer. Introduction The following is a brief description of a double beam spectrophotometer. The purpose of this instrument is to determine the amount of light of a specific wavelength absorbed by an analyte in a sample. Although samples can be gases or liquids, an analyte dissolved in a solvent is discussed here. [In the infrared, solid pellets using an IR. transparent matrix (like a high purity salt such as Kr) can be used for solid analytes. Thin disks are made using a pellet press and the disk suspended in the sample cell through which the sample beam passes.] The starting point in our movie is the light source. Depending on the wavelength of interest, this can be an electrically powered ultraviolet, visible, or infrared lamp. Not shown in the animations that accompany this page is the spectrophotometer's monochromator which selects the analytical wavelength from the source lamp's broad spectrum containing many wavelengths of light. The analytical wavelength is chosen based on the absorbance characteristics of the analyte. Monochromators are instruments whose sole purpose is to allow polychromatic (that is many wavelength containing) light into the entrance slit of the monochromator and only allow a single (or at least very few) wavelength (monochromatic light) out via the exit slit. This exiting, well-shaped, narrowly-defined beam now contains a small region of the electromagnetic spectrum. The spread, or band-pass, of the wavelengths depends on the slit settings of the monochromators (usually adjustable) and the quality of the light dispersing element in the monochromator (usually a grating in most modern monochromators). -

What-Is -Ft-Ir.Pdf
What is FT‐IR? Infrared (IR) spectroscopy is a chemical analytical technique, which measures the infrared intensity versus wavelength (wavenumber) of light. Based upon the wavenumber, infrared light can be categorized as far infrared (4 ~ 400cm‐1), mid infrared (400 ~ 4,000cm‐1) and near infrared (4,000 ~ 14,000cm‐1). Infrared spectroscopy detects the vibration characteristics of chemical functional groups in a sample. When an infrared light interacts with the matter, chemical bonds will stretch, contract and bend. As a result, a chemical functional group tends to adsorb infrared radiation in a specific wavenumber range regardless of the structure of the rest of the molecule. For example, the C=O stretch of a carbonyl group appears at around 1700cm‐1 in a variety of molecules. Hence, the correlation of the band wavenumber position with the chemical structure is used to identify a functional group in a sample. The wavenember positions where functional groups adsorb are consistent, despite the effect of temperature, pressure, sampling, or change in the molecule structure in other parts of the molecules. Thus the presence of specific functional groups can be monitored by these types of infrared bands, which are called group wavenumbers. The early‐stage IR instrument is of the dispersive type, which uses a prism or a grating monochromator. The dispersive instrument is characteristic of a slow scanning. A Fourier Transform Infrared (FTIR) spectrometer obtains infrared spectra by first collecting an interferogram of a sample signal with an interferometer, which measures all of infrared frequencies simultaneously. An FTIR spectrometer acquires and digitizes the interferogram, performs the FT function, and outputs the spectrum. -

Model 272 High Throughput Monochromator
Model 272 High Throughput Monochromator GENERAL DESCRIPTION The Model 272 monochromator contains a large, 100mm diameter diffraction grating. This guar- antees a large solid angle of light collection. The gratings corrected design reduces optical aberra- tions. This improves the spectral resolution of this high throughput optical system. The Model 272 is a versatile research grade monochromator. It delivers high throughput and moderate spectral resolution. Usable in any attitude, standard features of much larger McPherson monochromators have found home in this high effi ciency f/2 system. The Model 272 opto-mechanical system is ruggedly constructed for use in any environment. The lapped wavelength drive screw will provide years of accu- rate wavelength positioning. The grating scans are controlled by the optional Model 789A-3 digital scan drive system. McPherson signal recovery and control Model 272 High Throughput f/2 Monochromator software can be used. Most McPherson accesso- Aberration Corrected Grating Design ries work with this instrument. It is best to inquire to make sure the f/2 beams work at full capacity. Fast f/2 Optical System Wavelength Range from 185nm to NIR ARRAY DETECTION Aberration Corrected Grating Use for Scanning or Array Detection The Model 272 uses a concave grating that focuses and disperses light. The grating has aberration Optional Fiber Accessories correction to minimize spherical aberration and Compact, Lightweight Construction astigmatism. When used with a focal plane array Operates in any attitude the detector must be brought to the instrument focal plane that forms along a modifi ed Rowland circle. There may be mechanical, width and mounting re- SPECIFICATIONS strictions so please contact us to discuss the options. -

A Monochromator Is to Produce a Single Spectral Line from a Broadband (Multi-Wavelength) Source
Tunable Monochromator Introduction The job of a monochromator is to produce a single spectral line from a broadband (multi-wavelength) source. In spectrom- eters, this can be used to collect light from an atomic emission source, like the atomic emission detector, and allow only a specific line to exit. It can also be used to isolate a single line from a light source such as a hollow cathode lamp. The simple monochromator shown here is called a Czerny-Turner mono- chromator; however, other types are common. Source The instrument described here assumes that the input light is from a white light (a broadband source); although, many different inputs from which individual lines can be isolated are possible: a flame along with a hollow cathode lamp—as in AAS, a plasma—as in an atomic absorption spec- trometer, an ultraviolet source—as in a UV/Vis spectrometer, or in a fluorescence spectrometer, emission from a fluorescing sample. Entrance and Exit slits The purpose of the two slits in this monochromator is to control the size and “position” of the beam of light passing through the slit. On the way in, the entrance slit makes sure that only a small area of the input beam passes into the monochromator and that the light waves are relatively paral- lel coming from the source. Since the light will be carefully allowed to shine (flow?) among the mirrors and a grating inside the monochromator, parallel beams insure alignment of the light beams with the internal optics and cut down on stray light that might end up where it’s not wanted. -
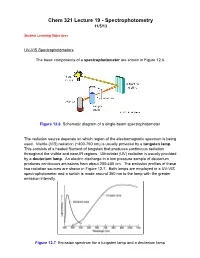
Chem 321 Lecture 19 - Spectrophotometry 11/5/13
Chem 321 Lecture 19 - Spectrophotometry 11/5/13 Student Learning Objectives UV-VIS Spectrophotometers The basic components of a spectrophotometer are shown in Figure 12.6. Figure 12.6 Schematic diagram of a single-beam spectrophotometer The radiation source depends on which region of the electromagnetic spectrum is being used. Visible (VIS) radiation (~400-750 nm) is usually provided by a tungsten lamp. This consists of a heated filament of tungsten that produces continuous radiation throughout the visible and near-IR regions. Ultraviolet (UV) radiation is usually provided by a deuterium lamp. An electric discharge in a low pressure sample of deuterium produces continuous emissions from about 200-400 nm. The emission profiles of these two radiation sources are shown in Figure 12.7. Both lamps are employed in a UV-VIS spectrophotometer and a switch is made around 360 nm to the lamp with the greater emission intensity. Figure 12.7 Emission spectrum for a tungsten lamp and a deuterium lamp Spectrophotometry 11/5/13 page 2 Since these sources are continuous, a small range of wavelengths must be selected for passage through the sample in order for Beer’s law to apply. This is accomplished by the monochromator. As diagramed in Figure 12.8, continuous radiation from the source enters the monochromator through a slit and the radiation reflects off a diffraction grating where it is dispersed (spread out). After focusing, only a small band of radiation emerges from the exit slit of the monochromator. By varying the orientation of the diffraction grating with respect to the incoming radiation, a different wavelength can be selected for passage through the monochromator. -

Lesson 1: X-Rays and Diffraction
Lesson 1 X-rays & Diffraction Nicola Döbelin RMS Foundation, Bettlach, Switzerland October 16 – 17, 2013, Uppsala, Sweden Electromagnetic Spectrum X rays: Wavelength λ: 0.01 – 10 nm Energy: 100 eV – 100 keV Interatomic distances in crystals: Generation of X-radiation: typically 0.15 – 0.4 nm Shoot electrons on matter Interference phenomena only for features ≈ λ 2 Generation of X-rays Accelerated electron impinges on matter: Bremsstrahlung (Deceleration radiation) Electron is deflected and decelerated by the atomic nucleus. (Inelastic scattering) Deflected electron emits electromagnetic radiation. Wavelength depends on the loss of energy. 3 Bremsstrahlung Continuous spectrum 40 kV, 20 mA 30 kV, 20 mA Intensity 20 kV, 20 mA 0.00 0.05 0.10 0.15 0.20 0.25 0.30 Wavelength (nm) 4 Bremsstrahlung Continuous spectrum 30 kV, 40 mA 30 kV, 30 mA Intensity 30 kV, 20 mA 0.00 0.05 0.10 0.15 0.20 0.25 0.30 Wavelength (nm) 5 Characteristic Radiation Cu Eb (eV) 0 M4,5 (3d) M2,3 (3p) 76 M1 (3s) 122 M L K L3 (2p 3/2 ) 933 952 L1 (2p 1/2 ) L1 (2s) 1097 Kα1 Kα2 Kβ K1 (1s) 8979 Wavelength of K α1, K α2, K β, L α... are characteristic for the atomic species. 6 X-rays: Spectrum Kα1 Kα1 Kα2 Kα2 Kβ Kβ Intensity Mo Cu 0.00 0.05 0.10 0.15 0.20 0.25 0.30 Wavelength (nm) 7 X-ray Tube Target (Cu, Mo, Fe, Co, ...) ‒ Acceleration e Be window Voltage Vacuum Filament Filament Current Generator settings: kV mA 8 Old X-ray tubes Lifetime of a few years: - Vacuum decreases loss of intensity - Tungsten from filament deposits on target contaminated spectrum (characteristic -

Development of Raman Spectrophotometer
(2>A^}jl <Uil aJJW Sudan Academy of Sciences, SAS Council of Engineering Research and Industrial Technology Development of Raman Spectrophotometer By A1AKHIB IBRAHIM ADAM (B.Sc. Honours in Electronics 2003) University of Elneelian A thesis submitted to the Sudan Academy of Sciences in fulfillment for the requirements for Master of Science in Electronic Engineering Supervisor Dr. El- Siddig Tawer Kafi May 2008 Development of Raman Spectrophotometer By ALAKHIB IBRAHIM ADAM Rxamination Committee Name Title Signature Dr. Mubark Clmahal External lixaminer Dr. Relal Kabashi Internal Examiner Dr. Siddig Tawer Supervisor Date of Examination 21/05/2008 ~±JS (j^kjll M ^ 53: AJV! cjkaa 1 DEDICATION THIS THESIS IS DEDICTED WITH LOVE TO THE SOUL OF MY MOTHER 11 AKNOWLEGENT With a deep sense of gratitude, I would like to express my sincere thanks to my supervisor Dr.Siddig. T. Kafi for his expert guidance, availability and continuous support during the work of the research. I thank Dr.A.Artolui for his valuable suggestions and technical support. Also I would like to thank Dr.rbrahim .Alimam for his helpful in the electronics sections. Also I would like to express my thanks to Mrs. Abdel-Sahki for his assistance during my lab work. Mostly, I extend my thanks to all my family and friends for their underlying support and encouragements. Finally, I thank every one contributed to making this research possible. in ABSTRACT In this work, the Raman spectrophotometer HG.2S Jobin Yvon rebuilt and developed, the Raman setup provided as a gift for Neelian University from Amsterdam University. The main parts, which were replaced, include monochromator, an air-cooled photomultiplier tube RCA IP 28, log amplifier, handscanning, labVIEW card for computer interfacing.