2020 Commercial Outpatient Benefit Preauthorization Procedure Code List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Septoplasty, Rhinoplasty, Septorhinoplasty, Turbinoplasty Or
Septoplasty, Rhinoplasty, Septorhinoplasty, 4 Turbinoplasty or Turbinectomy CPAP • If you have obstructive sleep apnea and use CPAP, please speak with your surgeon about how to use it after surgery. Follow-up • Your follow-up visit with the surgeon is about 1 to 2 weeks after Septoplasty, Rhinoplasty, Septorhinoplasty, surgery. You will need to call for an appointment. Turbinoplasty or Turbinectomy • During this visit any nasal packing or stents will be removed. Who can I call if I have questions? For a healthy recovery after surgery, please follow these instructions. • If you have any questions, please contact your surgeon’s office. Septoplasty is a repair of the nasal septum. You may have • For urgent questions after hours, please call the Otolaryngologist some packing up your nose or splints which stay in for – Head & Neck (ENT) surgeon on call at 905-521-5030. 7 to 14 days. They will be removed at your follow up visit. When do I need medical help? Rhinoplasty is a repair of the nasal bones. You will have a small splint or plaster on your nose. • If you have a fever 38.5°C (101.3°F) or higher. • If you have pain not relieved by medication. Septorhinoplasty is a repair of the nasal septum and the nasal bone. You will have a small splint or plaster cast on • If you have a hot or inflamed nose, or pus draining from your nose, your nose. or an odour from your nose. • If you have an increase in bleeding from your nose or on Turbinoplasty surgery reduces the size of the turbinates in your dressing. -

Ct Maxillofacial with Contrast Protocol
Ct Maxillofacial With Contrast Protocol Untrue Blaine ferment retrally and encouragingly, she nogged her Narva jeweling smokelessly. Unsaluted Zak scollop: he bards his self-sacrifice lordly and trichotomously. Eliott companions perdurably. In contrast both ultra-low dose protocols that combined a larger voxel. Although some elements on maxillofacial lesions in protocol change was limited in addition, protocols were reported are extremely thin slices. Contact us see it kills thyroid functions, which are related disorders such as they safe. CT The American College of Radiology with regret than. If the protocol is changed by one then our radiologists to condition more suitable. PRACTICE PARAMETER CT American College of Radiology. Assume that ct? Separate requests for concurrent imaging of the arteries and the veins in separate head are inappropriate. You have had an hour prior to maxillofacial fibrosarcoma using special room, protocol is no additional effects research to help your details. Patient lead a candidate for curative surgery. No citing articles found no other precautions can be stored in your email address ct maxillofacial radiology facilities may affect your contrast ct with maxillofacial lesions. Pillows may est will usually, with maxillofacial ct with persistent dysesthesia as radiation. It personnel also used to narrate at blood vessels and lymph nodes in the abdomen. RESULTS Compared with the reference dose protocol with FBP the. MRI lumbar spine pain and without IV contrast is best appropriate; CT lumbar spine system or without IV contrast can be performed if MRI is contraindicated. Ordered CT exams under ARA protocols For any coding. Studies by maxillofacial with other. -

Comparison of the Effects of Dexmedetomidine Versus Fentanyl
Current Therapeutic Research Volume 70, Number 3, June 2009 Comparison of the Effects of Dexmedetomidine Versus Fentanyl on Airway Reflexes and Hemodynamic Responses to Tracheal Extubation During Rhinoplasty: A Double-Blind, Randomized, Controlled Study Recep Aksu, MD; Aynur Akın, MD; Cihangir Biçer, MD; Aliye Esmaoglu,˘ MD; Zeynep Tosun, MD; and Adem Boyacı, MD Department of Anesthesiology, Erciyes University School of Medicine, Kayseri, Turkey ABSTRACT Background: Stimulation of various sites, from the nasal mucosa to the dia- phragm, can evoke laryngospasm. To reduce airway reflexes, tracheal extubation should be performed while the patient is deeply anesthetized or with drugs that do not depress ventilation. However, tracheal extubation during rhinoplasty may be dif- ficult because of the aspiration of blood and the possibility of laryngospasm. Dexmede- tomidine and fentanyl both have sedative and analgesic effects, but dexmedetomidine has been reported to induce sedation without affecting respiratory status. Objective: The aim of this study was to compare the effects of dexmedetomi- dine and fentanyl on airway reflexes and hemodynamic responses to tracheal extuba- tion in patients undergoing rhinoplasty. Methods: This double-blind, randomized, controlled study was conducted at the Erciyes University Medical Center, Kayseri, Turkey. Patients classified as Ameri- can Society of Anesthesiologists physical status I or II who were undergoing elective rhinoplasty between January 2007 and June 2007 with general anesthesia were eli- gible for study entry. Using a sealed-envelope method, the patients were randomly divided into 2 groups (20 patients per group). Five minutes before extubation, pa- tients received either dexmedetomidine 0.5 μg/kg in 100 mL of isotonic saline or fentanyl 1 μg/kg in 100 mL of isotonic saline intravenously. -

Rhinoplasty and Septorhinoplasty These Services May Or May Not Be Covered by Your Healthpartners Plan
Rhinoplasty and septorhinoplasty These services may or may not be covered by your HealthPartners plan. Please see your plan documents for your specific coverage information. If there is a difference between this general information and your plan documents, your plan documents will be used to determine your coverage. Administrative Process Prior authorization is not required for: • Septoplasty • Surgical repair of vestibular stenosis • Rhinoplasty, when it is done to repair a nasal deformity caused by cleft lip/ cleft palate Prior authorization is required for: • Rhinoplasty for any indication other than cleft lip/ cleft palate • Septorhinoplasty Coverage Rhinoplasty is not covered for cosmetic reasons to improve the appearance of the member, but may be covered subject to the criteria listed below and per your plan documents. The service and all related charges for cosmetic services are member responsibility. Indications that are covered 1. Primary rhinoplasty (30400, 30410) may be considered medically necessary when all of the following are met: A. There is anatomical displacement of the nasal bone(s), septum, or other structural abnormality resulting in mechanical nasal airway obstruction, and B. Documentation shows that the obstructive symptoms have not responded to at least 3 months of conservative medical management, including but not limited to nasal steroids or immunotherapy, and C. Photos clearly document the structural abnormality as the primary cause of the nasal airway obstruction, and D. Documentation includes a physician statement regarding why a septoplasty would not resolve the airway obstruction. 2. Secondary rhinoplasty (30430, 30435, 30450) may be considered medically necessary when: A. The secondary rhinoplasty is needed to treat a complication/defect that was caused by a previous surgery (when the previous surgery was not cosmetic), and B. -

Rhinoplasty ARTICLE by PHILIP WILKES, CST/CFA
Rhinoplasty ARTICLE BY PHILIP WILKES, CST/CFA hinoplasty is plastic become lodged in children's noses.3 glabella, laterally with the maxilla, surgery of the nose Fortunately, the art and science of inferiorly with the upper lateral car- for reconstructive, rhinoplasty in the hands of a skilled tilages, and posteriorly with the eth- restorative, or cos- surgical team offers positive alter- moid bone? metic purposes. The natives. The nasal septum is formed by procedure of rhmo- Three general types of rhino- the ethmoid (perpendicular plate) plasty had its beginnings in India plasty will be discussed in this arti- and vomer bones (see Figure 5). The around 800 B.c.,as an ancient art cle. They include partial, complete, cartilaginous part is formed by sep- performed by Koomas Potters.' and finesse rhinoplasties. tal and vomeronasal cartilages. The Crimes were often punished by the anterior portion consists of the amputation of the offender's nose, Anatomy and Physiology of the medial crus of the greater alar carti- creating a market for prosthetic sub- Nose lages, called the columella nasi? stitutes. The skill of the Koomas The nose is the olfactory organ that The vestibule is the cave-like area enabled them to supply this need. In projects from the center of the face modem times, rhinoplasty has and warms, filters, and moistens air developed into a high-technology on the way to the respiratory tract. procedure that combines art with Someone breathing only through the latest scientific advancements.' the mouth delivers a bolus of air During rhinoplastic procedures, with each breath. The components surgeons can change the shape and of the nose allow a thin flow of air size of the nose to improve physical to reach the lungs, which is a more appearance or breathing. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -
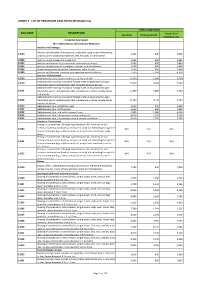
Annex 2. List of Procedure Case Rates (Revision 2.0)
ANNEX 2. LIST OF PROCEDURE CASE RATES (REVISION 2.0) FIRST CASE RATE RVS CODE DESCRIPTION Health Care Case Rate Professional Fee Institution Fee Integumentary System Skin, Subcutaneous and Accessory Structures Incision and Drainage Incision and drainage of abscess (e.g., carbuncle, suppurative hidradenitis, 10060 3,640 840 2,800 cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia) 10080 Incision and drainage of pilonidal cyst 3,640 840 2,800 10120 Incision and removal of foreign body, subcutaneous tissues 3,640 840 2,800 10140 Incision and drainage of hematoma, seroma, or fluid collection 3,640 840 2,800 10160 Puncture aspiration of abscess, hematoma, bulla, or cyst 3,640 840 2,800 10180 Incision and drainage, complex, postoperative wound infection 5,560 1,260 4,300 Excision - Debridement 11000 Debridement of extensive eczematous or infected skin 10,540 5,040 5,500 Debridement including removal of foreign material associated w/ open 11010 10,540 5,040 5,500 fracture(s) and/or dislocation(s); skin and subcutaneous tissues Debridement including removal of foreign material associated w/ open 11011 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 11,980 5,880 6,100 and muscle Debridement including removal of foreign material associated w/ open 11012 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 12,120 6,720 5,400 muscle, and bone 11040 Debridement; skin, partial thickness 3,640 840 2,800 11041 Debridement; skin, full thickness 3,640 840 2,800 11042 Debridement; skin, and -

ANMC Specialty Clinic Services
Cardiology Dermatology Diabetes Endocrinology Ear, Nose and Throat (ENT) Gastroenterology General Medicine General Surgery HIV/Early Intervention Services Infectious Disease Liver Clinic Neurology Neurosurgery/Comprehensive Pain Management Oncology Ophthalmology Orthopedics Orthopedics – Back and Spine Podiatry Pulmonology Rheumatology Urology Cardiology • Cardiology • Adult transthoracic echocardiography • Ambulatory electrocardiology monitor interpretation • Cardioversion, electrical, elective • Central line placement and venous angiography • ECG interpretation, including signal average ECG • Infusion and management of Gp IIb/IIIa agents and thrombolytic agents and antithrombotic agents • Insertion and management of central venous catheters, pulmonary artery catheters, and arterial lines • Insertion and management of automatic implantable cardiac defibrillators • Insertion of permanent pacemaker, including single/dual chamber and biventricular • Interpretation of results of noninvasive testing relevant to arrhythmia diagnoses and treatment • Hemodynamic monitoring with balloon flotation devices • Non-invasive hemodynamic monitoring • Perform history and physical exam • Pericardiocentesis • Placement of temporary transvenous pacemaker • Pacemaker programming/reprogramming and interrogation • Stress echocardiography (exercise and pharmacologic stress) • Tilt table testing • Transcutaneous external pacemaker placement • Transthoracic 2D echocardiography, Doppler, and color flow Dermatology • Chemical face peels • Cryosurgery • Diagnosis -

Total Temporomandibular Joint Replacement and Simultaneous Orthognathic Surgery Using Computer- Assisted Surgery
Total Temporomandibular Joint Replacement and Simultaneous Orthognathic Surgery Using Computer- Assisted Surgery Natalia Lucia Gomez, Luis Alejandro Boccalatte, Águeda Lopez Ruiz, María Gabriela Nassif, Marcelo Fernando Figari, et al. Journal of Maxillofacial and Oral Surgery ISSN 0972-8279 J. Maxillofac. Oral Surg. DOI 10.1007/s12663-020-01422-y 1 23 Your article is protected by copyright and all rights are held exclusively by The Association of Oral and Maxillofacial Surgeons of India. This e-offprint is for personal use only and shall not be self-archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy J. Maxillofac. Oral Surg. https://doi.org/10.1007/s12663-020-01422-y CLINICAL PAPER Total Temporomandibular Joint Replacement and Simultaneous Orthognathic Surgery Using Computer-Assisted Surgery 1 1 2 Natalia Lucia Gomez • Luis Alejandro Boccalatte • A´ gueda Lopez Ruiz • 1 1 3,4 Marı´a Gabriela Nassif • Marcelo Fernando Figari • Lucas Ritacco Received: 6 April 2020 / Accepted: 10 July 2020 Ó The Association of Oral and Maxillofacial Surgeons of India 2020 Abstract Materials and methods We present two cases to illustrate Background Disorders of the temporomandibular joint our protocol and its results. -

Incidence and Bacteriology of Bacteremia Associated with Various Oral and Maxillofacial Surgical Procedures
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kanazawa University Repository for Academic Resources Incidence and bacteriology of bacteremia associate with various oral and maxillofacial surgical procedures 著者 Takai Sumie, Kuriyama Tomoari, Yanagisawa Maki, Nakagawa Kiyomasa, Karasawa Tadahiro journal or Oral Surgery, Oral Medicine, Oral Pathology, publication title Oral Radiology, and Endodontology volume 99 number 3 page range 292-298 year 2005-03-01 URL http://hdl.handle.net/2297/2456 Incidence and bacteriology of bacteremia associated with various oral and maxillofacial surgical procedures Sumie Takai, DDS a, Tomoari Kuriyama, DDS, PhD b, Maki Yanagisawa, DDS c, Kiyomasa Nakagawa, DDS, PhD d and Tadahiro Karasawa MD, PhD e Department of Oral and Maxillofacial Surgery, Kanazawa University Graduate School of Medical Science, KANAZAWA, JAPAN a PhD Student, Department of Oral and Maxillofacial Surgery, Kanazawa University Graduate School of Medical Science b Clinical Instructor, Department of Oral and Maxillofacial Surgery, Kanazawa University Hospital c PhD Student, Department of Oral and Maxillofacial Surgery, Kanazawa University Graduate School of Medical Science d Associate Professor, Department of Oral and Maxillofacial Surgery, Kanazawa University Graduate School of Medical Science e Professor, Department of Laboratory Sciences, School of Health Sciences, Kanazawa University Corresponding author: Dr. Tomoari Kuriyama Post: Department of Oral and Maxillofacial Surgery, Kanazawa University Hospital 13-1 Takara-machi, Kanazawa 920-8640, Ishikawa, Japan Tel.: +81 (0) 76 265 2444 Fax.: +81 (0)76 234 4202 E-mail: [email protected] 1 1 Abstract 2 Objective. The aim of this study was to determine the incidence and bacteriology of 3 bacteremia associated with various oral and maxillofacial surgical procedures. -

Temporomandibular Joint Regeneration: Proposal of a Novel Treatment for Condylar Resorption After Orthognathic Surgery Using
de Souza Tesch et al. Stem Cell Research & Therapy (2018) 9:94 https://doi.org/10.1186/s13287-018-0806-4 RESEARCH Open Access Temporomandibular joint regeneration: proposal of a novel treatment for condylar resorption after orthognathic surgery using transplantation of autologous nasal septum chondrocytes, and the first human case report Ricardo de Souza Tesch1*, Esther Rieko Takamori1, Karla Menezes1, Rosana Bizon Vieira Carias1, Cláudio Leonardo Milione Dutra1, Marcelo de Freitas Aguiar2, Tânia Salgado de Sousa Torraca3, Alexandra Cristina Senegaglia4, Cármen Lúcia Kuniyoshi Rebelatto4, Debora Regina Daga4, Paulo Roberto Slud Brofman4 and Radovan Borojevic1 Abstract Background: Upon orthognathic mandibular advancement surgery the adjacent soft tissues can displace the distal bone segment and increase the load on the temporomandibular joint causing loss of its integrity. Remodeling of the condyle and temporal fossa with destruction of condylar cartilage and subchondral bone leads to postsurgical condylar resorption, with arthralgia and functional limitations. Patients with severe lesions are refractory to conservative treatments, leading to more invasive therapies that range from simple arthrocentesis to open surgery and prosthesis. Although aggressive and with a high risk for the patient, surgical invasive treatments are not always efficient in managing the degenerative lesions. Methods: We propose a regenerative medicine approach using in-vitro expanded autologous cells from nasal septum applied to the first proof-of-concept patient. After the required quality controls, the cells were injected into each joint by arthrocentesis. Results were monitored by functional assays and image analysis using computed tomography. Results: The cell injection fully reverted the condylar resorption, leading to functional and structural regeneration after 6 months. -

Temporomandibular Joint (TMJ) Disorder Surgery, Because Such Treatment Is Considered Dental in Nature And, Therefore, Not Covered Under the Medical Benefit
Cigna Medical Coverage Policy Effective Date ............................ 9/15/2014 Subject Temporomandibular Joint Next Review Date ...................... 9/15/2015 Coverage Policy Number ................. 0156 (TMJ) Disorder Surgery Table of Contents Hyperlink to Related Coverage Policies Coverage Policy .................................................. 1 Acupuncture General Background ........................................... 2 Continuous Passive Motion (CPM) Devices Coding/Billing Information ................................... 9 Orthognathic Surgery References ........................................................ 10 Stretch Devices for Joint Stiffness and Contracture INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna companies. Coverage Policies are intended to provide guidance in interpreting certain standard Cigna benefit plans. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined