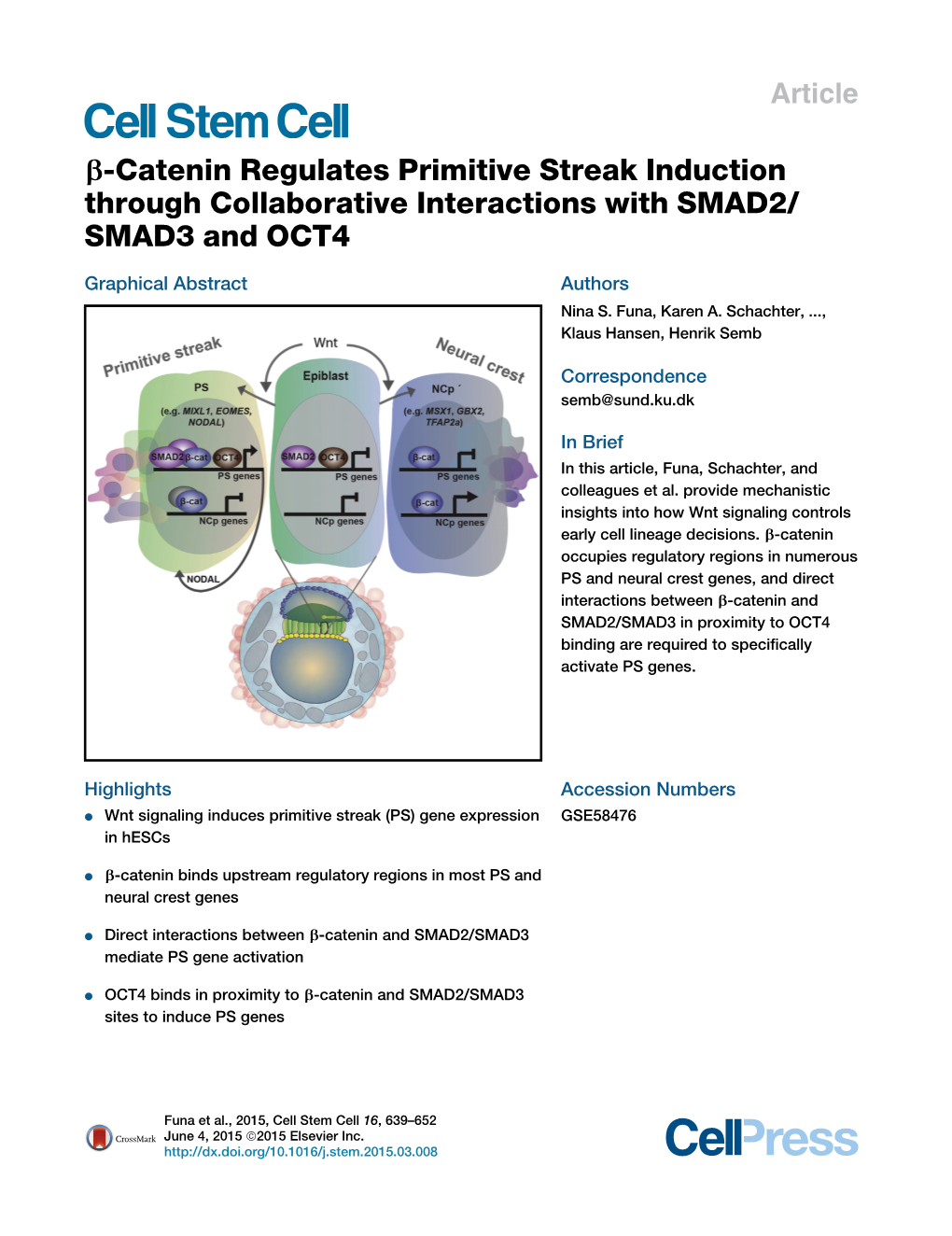
B-Catenin Regulates Primitive Streak Induction Through Collaborative Interactions with SMAD2/ SMAD3 and OCT4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downloaded from the National Database for Autism Research (NDAR)
International Journal of Molecular Sciences Article Phenotypic Subtyping and Re-Analysis of Existing Methylation Data from Autistic Probands in Simplex Families Reveal ASD Subtype-Associated Differentially Methylated Genes and Biological Functions Elizabeth C. Lee y and Valerie W. Hu * Department of Biochemistry and Molecular Medicine, The George Washington University, School of Medicine and Health Sciences, Washington, DC 20037, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-202-994-8431 Current address: W. Harry Feinstone Department of Molecular Microbiology and Immunology, y Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA. Received: 25 August 2020; Accepted: 17 September 2020; Published: 19 September 2020 Abstract: Autism spectrum disorder (ASD) describes a group of neurodevelopmental disorders with core deficits in social communication and manifestation of restricted, repetitive, and stereotyped behaviors. Despite the core symptomatology, ASD is extremely heterogeneous with respect to the severity of symptoms and behaviors. This heterogeneity presents an inherent challenge to all large-scale genome-wide omics analyses. In the present study, we address this heterogeneity by stratifying ASD probands from simplex families according to the severity of behavioral scores on the Autism Diagnostic Interview-Revised diagnostic instrument, followed by re-analysis of existing DNA methylation data from individuals in three ASD subphenotypes in comparison to that of their respective unaffected siblings. We demonstrate that subphenotyping of cases enables the identification of over 1.6 times the number of statistically significant differentially methylated regions (DMR) and DMR-associated genes (DAGs) between cases and controls, compared to that identified when all cases are combined. Our analyses also reveal ASD-related neurological functions and comorbidities that are enriched among DAGs in each phenotypic subgroup but not in the combined case group. -

The Rotated Hypoblast of the Chicken Embryo Does Not Initiate an Ectopic Axis in the Epiblast
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 10733-10737, November 1995 Developmental Biology The rotated hypoblast of the chicken embryo does not initiate an ectopic axis in the epiblast ODED KHANER Department of Cell and Animal Biology, The Hebrew University, Jerusalem, Israel 91904 Communicated by John Gerhart, University of California, Berkeley, CA, July 14, 1995 ABSTRACT In the amniotes, two unique layers of cells, of the hypoblast and that the orientation of the streak and axis the epiblast and the hypoblast, constitute the embryo at the can be predicted from the polarity of the stage XIII epiblast. blastula stage. All the tissues of the adult will derive from the Still it was thought that the polarity of the epiblast is sufficient epiblast, whereas hypoblast cells will form extraembryonic for axial development in experimental situations, although in yolk sac endoderm. During gastrulation, the endoderm and normal development the polarity of the hypoblast is dominant the mesoderm of the embryo arise from the primitive streak, (5, 6). These reports (1-3, 5, 6), taken together, would imply which is an epiblast structure through which cells enter the that cells of the hypoblast not only have the ability to change interior. Previous investigations by others have led to the the fate of competent cells in the epiblast to initiate an ectopic conclusion that the avian hypoblast, when rotated with regard axis, but also have the ability to repress the formation of the to the epiblast, has inductive properties that can change the original axis in committed cells of the epiblast. At the same fate of competent cells in the epiblast to form an ectopic time, the results showed that the epiblast is not wholly depen- embryonic axis. -

Molecular Genetic Delineation of 2Q37.3 Deletion in Autism and Osteodystrophy: Report of a Case and of New Markers for Deletion Screening by PCR
UC Irvine UC Irvine Previously Published Works Title Molecular genetic delineation of 2q37.3 deletion in autism and osteodystrophy: report of a case and of new markers for deletion screening by PCR. Permalink https://escholarship.org/uc/item/83f0x61r Journal Cytogenetics and cell genetics, 94(1-2) ISSN 0301-0171 Authors Smith, M Escamilla, JR Filipek, P et al. Publication Date 2001 DOI 10.1159/000048775 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Original Article Cytogenet Cell Genet 94:15–22 (2001) Molecular genetic delineation of 2q37.3 deletion in autism and osteodystrophy: report of a case and of new markers for deletion screening by PCR M. Smith, J.R. Escamilla, P. Filipek, M.E. Bocian, C. Modahl, P. Flodman, and M.A. Spence Department of Pediatrics, University of California, Irvine CA (USA) Abstract. We recently studied a patient who meets criteria us to determine the parental origin of the deletion in our for autistic disorder and has a 2q37 deletion. Molecular cyto- patient. DNA from 8–13 unrelated individuals was used to genetic studies were carried out using DNA isolated from 22 determine heterozygosity estimates for these markers. We re- different 2q37 mapped BACs to more precisely define the view four genes deleted in our patient – genes whose known extent of the chromosome deletion. We also analyzed 2q37 functions and sites of expression in the brain and/or bone make mapped polymorphic markers. In addition DNA sequences of them candidates for involvement in autism and/or the osteo- BACs in the deletion region were scanned to identify microsa- dystrophy observed in patients with 2q37.3 deletions. -

Gastrulation
Embryology of the spine and spinal cord Andrea Rossi, MD Neuroradiology Unit Istituto Giannina Gaslini Hospital Genoa, Italy [email protected] LEARNING OBJECTIVES: LEARNING OBJECTIVES: 1) To understand the basics of spinal 1) To understand the basics of spinal cord development cord development 2) To understand the general rules of the 2) To understand the general rules of the development of the spine development of the spine 3) To understand the peculiar variations 3) To understand the peculiar variations to the normal spine plan that occur at to the normal spine plan that occur at the CVJ the CVJ Summary of week 1 Week 2-3 GASTRULATION "It is not birth, marriage, or death, but gastrulation, which is truly the most important time in your life." Lewis Wolpert (1986) Gastrulation Conversion of the embryonic disk from a bilaminar to a trilaminar arrangement and establishment of the notochord The three primary germ layers are established The basic body plan is established, including the physical construction of the rudimentary primary body axes As a result of the movements of gastrulation, cells are brought into new positions, allowing them to interact with cells that were initially not near them. This paves the way for inductive interactions, which are the hallmark of neurulation and organogenesis Day 16 H E Day 15 Dorsal view of a 0.4 mm embryo BILAMINAR DISK CRANIAL Epiblast faces the amniotic sac node Hypoblast Primitive pit (primitive endoderm) faces the yolk sac Primitive streak CAUDAL Prospective notochordal cells Dias Dias During -

The Genetic Basis of Mammalian Neurulation
REVIEWS THE GENETIC BASIS OF MAMMALIAN NEURULATION Andrew J. Copp*, Nicholas D. E. Greene* and Jennifer N. Murdoch‡ More than 80 mutant mouse genes disrupt neurulation and allow an in-depth analysis of the underlying developmental mechanisms. Although many of the genetic mutants have been studied in only rudimentary detail, several molecular pathways can already be identified as crucial for normal neurulation. These include the planar cell-polarity pathway, which is required for the initiation of neural tube closure, and the sonic hedgehog signalling pathway that regulates neural plate bending. Mutant mice also offer an opportunity to unravel the mechanisms by which folic acid prevents neural tube defects, and to develop new therapies for folate-resistant defects. 6 ECTODERM Neurulation is a fundamental event of embryogenesis distinct locations in the brain and spinal cord .By The outer of the three that culminates in the formation of the neural tube, contrast, the mechanisms that underlie the forma- embryonic (germ) layers that which is the precursor of the brain and spinal cord. A tion, elevation and fusion of the neural folds have gives rise to the entire central region of specialized dorsal ECTODERM, the neural plate, remained elusive. nervous system, plus other organs and embryonic develops bilateral neural folds at its junction with sur- An opportunity has now arisen for an incisive analy- structures. face (non-neural) ectoderm. These folds elevate, come sis of neurulation mechanisms using the growing battery into contact (appose) in the midline and fuse to create of genetically targeted and other mutant mouse strains NEURAL CREST the neural tube, which, thereafter, becomes covered by in which NTDs form part of the mutant phenotype7.At A migratory cell population that future epidermal ectoderm. -

Agonists Regulate the Expression of Stemness and Differentiation Genes in Brain Tumour Stem Cells
British Journal of Cancer (2012) 106, 1702–1712 & 2012 Cancer Research UK All rights reserved 0007 – 0920/12 www.bjcancer.com PPARg agonists regulate the expression of stemness and differentiation genes in brain tumour stem cells E Pestereva1, S Kanakasabai1 and JJ Bright*,1,2 1 Neuroscience Research Laboratory, Methodist Research Institute, Indiana University Health, 1800 North Capitol Avenue, Noyes Building E504C, 2 Indianapolis, IN 46202, USA; Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA BACKGROUND: Brain tumour stem cells (BTSCs) are a small population of cancer cells that exhibit self-renewal, multi-drug resistance, and recurrence properties. We have shown earlier that peroxisome proliferator-activated receptor gamma (PPARg) agonists inhibit the expansion of BTSCs in T98G and U87MG glioma. In this study, we analysed the influence of PPARg agonists on the expression of stemness and differentiation genes in BTSCs. METHODS: The BTSCs were isolated from T98G and DB29 glioma cells, and cultured in neurobasal medium with epidermal growth factor þ basic fibroblast growth factor. Proliferation was measured by WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2 H-5- tetrazolio]-1,3-benzene disulphonate) and 3H thymidine uptake assays, and gene expression was analysed by quantitative reverse– transcription PCR and Taqman array. The expression of CD133, SRY box 2, and nanog homeobox (Nanog) was also evaluated by western blotting, immunostaining, and flow cytometry. 12,14 RESULTS: We found that PPARg agonists, ciglitazone and 15-deoxy-D -ProstaglandinJ2, inhibited cell viability and proliferation of þ T98G- and DB29-BTSCs. The PPARg agonists reduced the expansion of CD133 BTSCs and altered the expression of stemness and differentiation genes. -

Understanding Paraxial Mesoderm Development and Sclerotome Specification for Skeletal Repair Shoichiro Tani 1,2, Ung-Il Chung2,3, Shinsuke Ohba4 and Hironori Hojo2,3
Tani et al. Experimental & Molecular Medicine (2020) 52:1166–1177 https://doi.org/10.1038/s12276-020-0482-1 Experimental & Molecular Medicine REVIEW ARTICLE Open Access Understanding paraxial mesoderm development and sclerotome specification for skeletal repair Shoichiro Tani 1,2, Ung-il Chung2,3, Shinsuke Ohba4 and Hironori Hojo2,3 Abstract Pluripotent stem cells (PSCs) are attractive regenerative therapy tools for skeletal tissues. However, a deep understanding of skeletal development is required in order to model this development with PSCs, and for the application of PSCs in clinical settings. Skeletal tissues originate from three types of cell populations: the paraxial mesoderm, lateral plate mesoderm, and neural crest. The paraxial mesoderm gives rise to the sclerotome mainly through somitogenesis. In this process, key developmental processes, including initiation of the segmentation clock, formation of the determination front, and the mesenchymal–epithelial transition, are sequentially coordinated. The sclerotome further forms vertebral columns and contributes to various other tissues, such as tendons, vessels (including the dorsal aorta), and even meninges. To understand the molecular mechanisms underlying these developmental processes, extensive studies have been conducted. These studies have demonstrated that a gradient of activities involving multiple signaling pathways specify the embryonic axis and induce cell-type-specific master transcription factors in a spatiotemporal manner. Moreover, applying the knowledge of mesoderm development, researchers have attempted to recapitulate the in vivo development processes in in vitro settings, using mouse and human PSCs. In this review, we summarize the state-of-the-art understanding of mesoderm development and in vitro modeling of mesoderm development using PSCs. We also discuss future perspectives on the use of PSCs to generate skeletal tissues for basic research and clinical applications. -

Sonic Hedgehog a Neural Tube Anti-Apoptotic Factor 4013 Other Side of the Neural Plate, Remaining in Contact with Midline Cells, RESULTS Was Used As a Control
Development 128, 4011-4020 (2001) 4011 Printed in Great Britain © The Company of Biologists Limited 2001 DEV2740 Anti-apoptotic role of Sonic hedgehog protein at the early stages of nervous system organogenesis Jean-Baptiste Charrier, Françoise Lapointe, Nicole M. Le Douarin and Marie-Aimée Teillet* Institut d’Embryologie Cellulaire et Moléculaire, CNRS FRE2160, 49bis Avenue de la Belle Gabrielle, 94736 Nogent-sur-Marne Cedex, France *Author for correspondence (e-mail: [email protected]) Accepted 19 July 2001 SUMMARY In vertebrates the neural tube, like most of the embryonic notochord or a floor plate fragment in its vicinity. The organs, shows discreet areas of programmed cell death at neural tube can also be recovered by transplanting it into several stages during development. In the chick embryo, a stage-matched chick embryo having one of these cell death is dramatically increased in the developing structures. In addition, cells engineered to produce Sonic nervous system and other tissues when the midline cells, hedgehog protein (SHH) can mimic the effect of the notochord and floor plate, are prevented from forming by notochord and floor plate cells in in situ grafts and excision of the axial-paraxial hinge (APH), i.e. caudal transplantation experiments. SHH can thus counteract a Hensen’s node and rostral primitive streak, at the 6-somite built-in cell death program and thereby contribute to organ stage (Charrier, J. B., Teillet, M.-A., Lapointe, F. and Le morphogenesis, in particular in the central nervous system. Douarin, N. M. (1999). Development 126, 4771-4783). In this paper we demonstrate that one day after APH excision, Key words: Apoptosis, Avian embryo, Cell death, Cell survival, when dramatic apoptosis is already present in the neural Floor plate, Notochord, Quail/chick, Shh, Somite, Neural tube, tube, the latter can be rescued from death by grafting a Spinal cord INTRODUCTION generally induces an inflammatory response. -

The Origin of Early Primitive Streak 89
Development 127, 87-96 (2000) 87 Printed in Great Britain © The Company of Biologists Limited 2000 DEV3080 Formation of the avian primitive streak from spatially restricted blastoderm: evidence for polarized cell division in the elongating streak Yan Wei and Takashi Mikawa* Department of Cell Biology, Cornell University Medical College, 1300 York Avenue, New York, NY 10021, USA *Author for correspondence (e-mail: [email protected]) Accepted 13 October; published on WWW 8 December 1999 SUMMARY Gastrulation in the amniote begins with the formation of a cells generated daughter cells that underwent a polarized primitive streak through which precursors of definitive cell division oriented perpendicular to the anteroposterior mesoderm and endoderm ingress and migrate to their embryonic axis. The resulting daughter cell population was embryonic destinations. This organizing center for amniote arranged in a longitudinal array extending the complete gastrulation is induced by signal(s) from the posterior length of the primitive streak. Furthermore, expression of margin of the blastodisc. The mode of action of these cVg1, a posterior margin-derived signal, at the anterior inductive signal(s) remains unresolved, since various marginal zone induced adjacent epiblast cells, but not those origins and developmental pathways of the primitive streak lateral to or distant from the signal, to form an ectopic have been proposed. In the present study, the fate of primitive streak. The cVg1-induced epiblast cells also chicken blastodermal cells was traced for the first time in exhibited polarized cell divisions during ectopic primitive ovo from prestreak stages XI-XII through HH stage 3, streak formation. These results suggest that blastoderm when the primitive streak is initially established and prior cells located immediately anterior to the posterior marginal to the migration of mesoderm. -

PDF Download
Review Xatzipsalti Maria et al. Congenital Hypopituitarism: Various Genes, … Horm Metab Res 2018; 00: 00–00 Congenital Hypopituitarism: Various Genes, Various Phenotypes Authors Maria Xatzipsalti1, 2, Antonis Voutetakis1, Lela Stamoyannou2, George P. Chrousos1, Christina Kanaka-Gantenbein1 Affiliations ABSTRacT 1 Division of Endocrinology, Diabetes and Metabolism, The ontogenesis and development of the pituitary gland is a First Department of Pediatrics, Medical School, National highly complex process that depends on a cascade of transcrip- and Kapodistrian University of Athens, “Aghia Sofia” tion factors and signaling molecules. Spontaneous mutations Children's Hospital, Athens, Greece and transgenic murine models have demonstrated a role for 2 First Department of Pediatrics, “Aglaia Kyriakou” many of these factors, including HESX1, PROP1, PIT1, LHX3, Children's Hospital, Athens, Greece LHX4, SOX2, SOX3, OTX2, PAX6, FGFR1, SHH, GLI2, and FGF8 in the etiology of congenital hypopituitarism. Genetic muta- Key words tions in any of these factors can lead to congenital hypopitui- pituitary, combined pituitary hormone deficiency, congenital tarism, which is characterized by the deficiency in one or more hypopituitarism, transcription factors, syndromic hypopitui- pituitary hormones. The phenotype can be highly variable, tarism, non-syndromic hypopituitarism consisting of isolated hypopituitarism or more complex disor- ders. The same phenotype can be attributed to different gene received 27.03.2018 mutations; while a given gene mutation can -

A Review of Primitive Streak Formation and Somitogenesis
SPATIOTEMPORAL PATTERN FORMATION IN EARLY DEVELOPMENT: A REVIEW OF PRIMITIVE STREAK FORMATION AND SOMITOGENESIS S. SCHNELL', K.J. PAINTERt, P.K. MAINI' , AND H.G. OTHMERt Abstract. The basic body plan of a number of vertebrates results from two pro cesses that occur early in the development of the blastoderm: large scale rearrangements of tissue via a process called gastrulation, and axial subdivision of tissue in a process called somitogenesis. The first step of gastrulation in avians is formation of the prim itive streak, which marks the first clear manifestation of the anterior-posterior axis. Cell movements that occur through the streak ultimately convert the single layered blastoderm into a trilaminar blastoderm comprising prospective endodermal, mesoder mal and ectodermal tissue. During streak formation a group of cells moves anteriorly as a coherent column from the posterior end of the blastoderm, and as it proceeds other cells stream over the lateral edges of the furrow left behind. The anterior end of the streak is a specialized structure called Hensen's node, which serves as an organizing center for later axis formation and determination of the left-right asymmetry of the body. Soon after the primitive streak forms, Hensen's node regresses towards the tail, leaving the notochord and a pair of segmental plates parallel to the primitive streak in its wake. The posterior end of the segmental plate moves down the cranio-caudal axis with the node, as more cells are added to it by cell division within the plate and by cells entering from the primitive streak. A pair of somites forms from the anterior ends of the two plates at regular intervals. -

FOS Rescues Neuronal Differentiation of Sox2-Deleted Neural Stem Cells by Genome-Wide Regulation of Common SOX2 and AP1(FOS-JUN) Target Genes
cells Article FOS Rescues Neuronal Differentiation of Sox2-Deleted Neural Stem Cells by Genome-Wide Regulation of Common SOX2 and AP1(FOS-JUN) Target Genes Miriam Pagin 1,† , Mattias Pernebrink 2,3,†, Mattia Pitasi 1, Federica Malighetti 1, Chew-Yee Ngan 4, Sergio Ottolenghi 1, Giulio Pavesi 5, Claudio Cantù 2,3,* and Silvia K. Nicolis 1,* 1 Department of Biotechnology and Biosciences, University of Milano-Bicocca, Piazza della Scienza 2, 20126 Milano, Italy; [email protected] (M.P.); [email protected] (M.P.); [email protected] (F.M.); [email protected] (S.O.) 2 Wallenberg Centre for Molecular Medicine, Linköping University, SE-581 83 Linköping, Sweden; [email protected] 3 Department of Biomedical and Clinical Sciences, Division of Molecular Medicine and Virology, Faculty of Medicine and Health Sciences, Linköping University, SE-581 83 Linköping, Sweden 4 The Jackson Laboratory for Genomic Medicine, Farmington, CT 06032, USA; [email protected] 5 Department of Biosciences, University of Milano, Via Celoria 26, 20134 Milano, Italy; [email protected] * Correspondence: [email protected] (C.C.); [email protected] (S.K.N.) † Joint first authors. Abstract: The transcription factor SOX2 is important for brain development and for neural stem Citation: Pagin, M.; Pernebrink, M.; cells (NSC) maintenance. Sox2-deleted (Sox2-del) NSC from neonatal mouse brain are lost after Pitasi, M.; Malighetti, F.; Ngan, C.-Y.; few passages in culture. Two highly expressed genes, Fos and Socs3, are strongly downregulated in Ottolenghi, S.; Pavesi, G.; Cantù, C.; Sox2-del NSC; we previously showed that Fos or Socs3 overexpression by lentiviral transduction fully Nicolis, S.K.