Table S1. Checklist Summarizing Compliance with PRISMA Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COVID-19 Vaccine Janssen: Contraindication in Individuals with Previous Capillary Leak Syndrome and Update on Thrombosis with Thrombocytopenia Syndrome
Janssen Sciences Ireland UC Address: Airton Road, Tallaght, D24 WR89 Tel: +353 1 466 5200 Fax: +353 1 431 1058 www.janssen.ie 19the July 2021 COVID-19 Vaccine Janssen: Contraindication in individuals with previous capillary leak syndrome and update on thrombosis with thrombocytopenia syndrome Dear Healthcare Professional, Janssen-Cilag International NV in agreement with the European Medicines Agency and the Health Products Regulatory Authority (HPRA) would like to inform you of the following: Summary Capillary leak syndrome (CLS): • Very rare cases of capillary leak syndrome (CLS) have been reported in the first days after vaccination with COVID-19 Vaccine Janssen, in some cases with a fatal outcome. A history of CLS has been reported in at least one case. • COVID-19 Vaccine Janssen is now contraindicated in individuals who have previously experienced episodes of CLS. • CLS is characterised by acute episodes of oedema mainly affecting the limbs, hypotension, haemoconcentration and hypoalbuminaemia. Patients with an acute episode of CLS following vaccination require prompt recognition and treatment. Intensive supportive therapy is usually warranted. Thrombosis with thrombocytopenia syndrome (TTS): • Individuals diagnosed with thrombocytopenia within 3 weeks after vaccination with COVID-19 Vaccine Janssen should be actively investigated for signs of thrombosis. Similarly, individuals who present with thrombosis within 3 weeks of vaccination should be evaluated for thrombocytopenia. • TTS requires specialised clinical management. Healthcare professionals should consult applicable guidance and/ or consult specialists (e.g., haematologists, specialists in coagulation) to diagnose and treat this condition. Background on the safety concern COVID-19 Vaccine Janssen suspension for injection is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older. -

Capillary Leak Syndrome
The Medicine Forum Volume 17 Article 8 2016 Case Report: Uncontrolled Anasarca: Capillary Leak Syndrome Ankita Mehta, MD Thomas Jefferson University Hospital, [email protected] Mansi Shah, MD Jefferson Hospital Ambulatory Practice, Thomas Jefferson University Hospital, [email protected] Follow this and additional works at: https://jdc.jefferson.edu/tmf Part of the Internal Medicine Commons, and the Oncology Commons Let us know how access to this document benefits ouy Recommended Citation Mehta, MD, Ankita and Shah, MD, Mansi (2016) "Case Report: Uncontrolled Anasarca: Capillary Leak Syndrome," The Medicine Forum: Vol. 17 , Article 8. DOI: https://doi.org/10.29046/TMF.017.1.009 Available at: https://jdc.jefferson.edu/tmf/vol17/iss1/8 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in The Medicine Forum by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Mehta, MD and Shah, MD: Case Report: Uncontrolled Anasarca: Capillary Leak Syndrome ONCOLOGY Case Report: Uncontrolled Anasarca: Capillary Leak Syndrome Ankita Mehta, MD, and Mansi Shah, MD INTRODUCTION progression of her pancreatic cancer. She was ultimately discharged home with hospice care. -

Common Terminology Criteria for Adverse Events
Common Terminology Criteria for Adverse Events Version 3.0 Index Cancer Therapy Evaluation Program NATIONAL INSTITUTES OF HEALTH National Cancer Institute http://ctep.cancer.gov/ 10/22/2003 Public Health Service Cancer Therapy Evaluation Program National Institutes of Health National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) - Index Terms Report Bethesda, Maryland 20892 -- A -- abdomen ENDOCRINE HEPATOBILIARY/PANCREAS Adrenal insufficiency 17 Liver dysfunction/failure (clinical) 34 GASTROINTESTINAL Pancreatitis 34 Colitis 19 PAIN Distension/bloating, abdominal 20 Pain --Select 55 Enteritis (inflammation of the small bowel) 21 VASCULAR Fistula, GI --Select 22 Portal vein flow 70 Typhlitis (cecal inflammation) 27 HEMORRHAGE/BLEEDING abducens Hemorrhage, GI --Select 31 See: CN VI HEPATOBILIARY/PANCREAS abnormal Pancreatitis 34 BLOOD/BONE MARROW INFECTION Myelodysplasia 4 Colitis, infectious (e.g., Clostridium difficile) 35 CONSTITUTIONAL SYMPTOMS Infection (documented clinically or microbiologically) with 35 Odor (patient odor) 11 Grade 3 or 4 neutrophils (ANC <1.0 x 10e9/L) --Select Infection with normal ANC or Grade 1 or 2 neutrophils -- 35 ENDOCRINE Select Neuroendocrine: 17 Infection with unknown ANC --Select 36 ADH secretion abnormality (e.g., SIADH or low ADH) Neuroendocrine: 17 MUSCULOSKELETAL/SOFT TISSUE gonadotropin secretion abnormality Soft tissue necrosis --Select 46 Neuroendocrine: 17 PAIN growth hormone secretion abnormality Neuroendocrine: 17 Pain --Select 55 prolactin hormone secretion abnormality -

Multistage Delivery of Active Agents
111111111111111111111111111111111111111111111111111111111111111111111111111111 (12) United States Patent (io) Patent No.: US 10,143,658 B2 Ferrari et al. (45) Date of Patent: Dec. 4, 2018 (54) MULTISTAGE DELIVERY OF ACTIVE 6,355,270 B1 * 3/2002 Ferrari ................. A61K 9/0097 AGENTS 424/185.1 6,395,302 B1 * 5/2002 Hennink et al........ A61K 9/127 (71) Applicants:Board of Regents of the University of 264/4.1 2003/0059386 Al* 3/2003 Sumian ................ A61K 8/0241 Texas System, Austin, TX (US); The 424/70.1 Ohio State University Research 2003/0114366 Al* 6/2003 Martin ................. A61K 9/0097 Foundation, Columbus, OH (US) 424/489 2005/0178287 Al* 8/2005 Anderson ............ A61K 8/0241 (72) Inventors: Mauro Ferrari, Houston, TX (US); 106/31.03 Ennio Tasciotti, Houston, TX (US); 2008/0280140 Al 11/2008 Ferrari et al. Jason Sakamoto, Houston, TX (US) FOREIGN PATENT DOCUMENTS (73) Assignees: Board of Regents of the University of EP 855179 7/1998 Texas System, Austin, TX (US); The WO WO 2007/120248 10/2007 Ohio State University Research WO WO 2008/054874 5/2008 Foundation, Columbus, OH (US) WO WO 2008054874 A2 * 5/2008 ............... A61K 8/11 (*) Notice: Subject to any disclaimer, the term of this OTHER PUBLICATIONS patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. Akerman et al., "Nanocrystal targeting in vivo," Proc. Nad. Acad. Sci. USA, Oct. 1, 2002, 99(20):12617-12621. (21) Appl. No.: 14/725,570 Alley et al., "Feasibility of Drug Screening with Panels of Human tumor Cell Lines Using a Microculture Tetrazolium Assay," Cancer (22) Filed: May 29, 2015 Research, Feb. -

Life-Threatening Capillary Leak Syndrome
on Tec ati hn nt o la lo p g s i e n s a r & Journal of Yi-Zhi et al., J Transplant Technol Res 2015, S4 T R f e o l s DOI: 10.4172/2161-0991.1000S4-003 a e a n ISSN:r 2161-0991 r c u h o J Transplantation Technologies & Research CaseResearch Report Article OpenOpen Access Access EditorialResearchCase Report Article OpenOpen Access Access Life-threatening Capillary Leak Syndrome in an Adult with Refractory Acute Myeloid Leukemia during Allogeneic Transplantation: a Case Report and Review of Literature Yi-Zhi J, Lai-Quan H, Gui-Ping S, Yan D, He-Sheng H and Dong-Ping H* Department of Hematology, The Affiliated Yijishan Hospital of Wannan Medical College, Wuhu, China Abstract Background: Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) offers the possibility of cure for hematological malignancies, various complications have been described. Capillary leak syndrome (CLS) has been previously observed in HSCT patients. CLS is a rare disease characterized by recurrent episodes of generalized edema and severe hypotension along with hypoproteinemia. Case Report: A 27-year-old Chinese man, diagnosed with refractory acute myeloid leukemia, was treated with a haploidentical stem cell transplant combined with an unrelated umbilical cord blood unit. The patient developed fatal CLS during the 9th day of the conditioning therapy. Conclusion: Since it is difficult to distinguish between CLS and other early complications during allo-HSCT, our report highlights the need for rigorous investigation of identifying CLS and the increasing need of insightful diagnosis to manage any incidence of CLS. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Necrotizing Soft-Tissue Infections
11/1/2020 Necrotizing Fasciitis ▪Is a surgical diagnosis characterized by friability of the superficial fascia, Necrotizing dishwater-gray exudate, and a notable Soft-Tissue absence of pus. Infections N ENGL J MED 2017;377:2253-65. 1 2 Necrotizing fasciitis type I: Necrotizing fasciitis type II ▪ Polymicrobial infection involving aerobic ▪ Often associated with gas in the tissue ▪ A monomicrobial infection. and anaerobic organisms. and thus is difficult to distinguish from gas gangrene ▪ Seen in the elderly or in those with ▪ Among gram-positive organisms, group A streptococcus is the underlying illnesses. ▪ Nonclostridial anaerobic cellulitis and most common pathogen, followed by MRSA. synergistic necrotizing cellulitis are type ▪ Predisposed I variants. Both occur in patients with ▪ May occur in any age group and in those without underlying illness ▪ Diabetic or decubitus ulcers diabetes and typically involve the feet, ▪ Hemorrhoids with rapid extension into the leg ▪ Aeromonas hydrophila (freshwater laceration) ▪ Rectal fissures ▪ Necrotizing fasciitis should be ▪ Vibrio vulnificus (saltwater laceration, ingestion of raw oysters, ▪ Episiotomies considered in patients with systemic cirrhosis) ▪ Colonic or urologic surgery or gynecologic manifestations of sepsis, such as procedures. tachycardia, leukocytosis, acidosis, or ▪ Necrotizing group A streptococcal and clostridial infections are marked hyperglycemia mediated by bacterial exotoxins and the host response ▪ Ludwigs- submandibular ▪ Lemierre’s –thrombophlebitis of jugular ▪ Fournier’s-urethral breach 3 4 Invasive Group A Streptococcal Soft-Tissue Infections Invasive Group A Streptococcal Soft-Tissue Infections (Streptococcus Continued pyogenes) ▪ Infection with a defined portal of bacterial entry: Infection that arises spontaneously in the deep tissue, without an overt wound or lesion: ▪ In 50% of patients with group A streptococcal necrotizing fasciitis or myonecrosis, are without a portal ▪ S. -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] BMJ Open Pediatric drug utilization in the Western Pacific region: Australia, Japan, South Korea, Hong Kong and Taiwan Journal: BMJ Open ManuscriptFor ID peerbmjopen-2019-032426 review only Article Type: Research Date Submitted by the 27-Jun-2019 Author: Complete List of Authors: Brauer, Ruth; University College London, Research Department of Practice and Policy, School of Pharmacy Wong, Ian; University College London, Research Department of Practice and Policy, School of Pharmacy; University of Hong Kong, Centre for Safe Medication Practice and Research, Department -

Signal AR on Embolic and Thrombotic Events with COVID-19 Vaccine
20th April 2021 EMA/268126/2021 Pharmacovigilance Risk Assessment Committee (PRAC) Signal assessment report on Embolic and Thrombotic events (SMQ) with COVID-19 Vaccine Janssen (Ad26.COV2-S [recombinant]) EPITT no:19689 Confirmation assessment report 3rd April 2021 Adoption of first PRAC Recommendation 9th April 2021 Submission of responses by MAH 15th April 2021 Preliminary assessment report 19th April 2021 Deadline for comments 19th April 2021 (8pm CET) Updated Rapporteur assessment 20th April 2021 (9am Adoption of 2nd PRAC recommendation 20th April 2021 Submission of responses by MAH 22nd April 2021 Rapporteur assessment 30th April 2021 Deadline for comments 30th April 2021 (8pm CET) Updated Rapporteur assessment report 3rd May 2021 Adoption of 3rd PRAC recommendation 06th May 2021 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Ne therla nds Address for visits and deliveries Refer to www.ema.europa.eu/how-to-fi nd-us An agency of the European Union Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 © Euro pe an Me dici ne s Age ncy, 2021. Reproduction is authorised provided the source is acknowledged. Administrative information Active substance(s) (invented name) COVID-19 Vaccine (Ad26.COV2-S [recombinant]) – COVID-19 Vaccine Janssen suspension for injection (Other viral vaccines) Strength(s) All Pharmaceutical form(s) All Route(s) of administration All Indication(s) COVID-19 Vaccine Janssen is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in individuals -
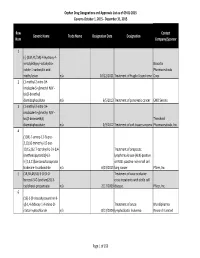
Orphan Drug Designation List
Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 1 (‐)‐(3aR,4S,7aR)‐4‐Hydroxy‐4‐ m‐tolylethynyl‐octahydro‐ Novartis indole‐1‐carboxylic acid Pharmaceuticals methyl ester n/a 10/12/2011 Treatment of Fragile X syndrome Corp. 2 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐broethyl) diamidophosphate n/a 6/5/2013 Treatment of pancreatic cancer EMD Serono 3 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐bromoethyl) Threshold diamidophosphate n/a 3/9/2012 Treatment of soft tissue sarcoma Pharmaceuticals, Inc. 4 (1OR)‐7‐amino‐12‐fluoro‐ 2,10,16‐trimethyl‐15 oxo‐ 10,15,16,17‐tetrahydro‐2H‐8,4‐ Treatment of anaplastic (metheno)pyrazolo[4,3‐ lymphoma kinase (ALK)‐positive h][2,5,11]benzoxadiazacyclote or ROS1‐positive non‐small cell tradecine‐3‐carbonitrile n/a 6/23/2015 lung cancer Pfizer, Inc. 5 (1R,3R,4R,5S)‐3‐O‐[2‐O‐ Treatment of vaso‐occlusive benzoyl‐3‐O‐(sodium(2S)‐3‐ crisis in patients with sickle cell cyclohexyl‐propanoate‐ n/a 2/17/2009 disease. Pfizer, Inc. 6 (1S)‐1‐(9‐deazahypoxanthin‐9‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ Treatment of acute Mundipharma ribitol‐hydrochloride n/a 8/13/2004 lymphoblastic leukemia Research Limited Page 1 of 359 Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 7 Treatment of chronic lymphocytic leukemia and related leukemias to include (1S)‐1‐(9‐deazahypoxanthin‐9‐ prolymphocytic leukemia, adult T‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ cell leukemia, and hairy cell Mundipharma ribitol‐hydrochloride n/a 8/10/2004 leukemia Research Ltd. -

Safety, Pharmacokinetics, Pharmacodynamics, and Antitumor Activity of Dalantercept, an Activin Receptor–Like Kinase-1 Ligand Trap, in Patients with Advanced Cancer
Published OnlineFirst October 30, 2013; DOI: 10.1158/1078-0432.CCR-13-1840 Clinical Cancer Cancer Therapy: Clinical Research Safety, Pharmacokinetics, Pharmacodynamics, and Antitumor Activity of Dalantercept, an Activin Receptor–like Kinase-1 Ligand Trap, in Patients with Advanced Cancer Johanna C. Bendell1, Michael S. Gordon2, Herbert I. Hurwitz3, Suzanne F. Jones1, David S. Mendelson2, Gerard C. Blobe3, Neeraj Agarwal4, Carolyn H. Condon5, Dawn Wilson5, Amelia E. Pearsall5, Yijun Yang5, Ty McClure5, Kenneth M. Attie5, Matthew L. Sherman5, and Sunil Sharma4 Abstract Purpose: The angiogenesis inhibitor dalantercept (formerly ACE-041) is a soluble form of activin receptor–like kinase-1 (ALK1) that prevents activation of endogenous ALK1 by bone morphogenetic protein-9 (BMP9) and BMP10 and exhibits antitumor activity in preclinical models. This first-in-human study of dalantercept evaluated its safety, tolerability, pharmacokinetics, pharmacodynamics, and antitu- mor activity in adults with advanced solid tumors. Experimental Design: Patients in dose-escalating cohorts received dalantercept subcutaneously at one of seven dose levels (0.1–4.8 mg/kg) every 3 weeks until disease progression. Patients in an expansion cohort received dalantercept at 0.8 or 1.6 mg/kg every 3 weeks until disease progression. Results: In 37 patients receiving dalantercept, the most common treatment-related adverse events were peripheral edema, fatigue, and anemia. Edema and fluid retention were dose-limiting toxicities and responded to diuretic therapy. No clinically significant, treatment-related hypertension, proteinuria, gross hemorrhage, or gastrointestinal perforations were observed. One patient with refractory squamous cell cancer of the head and neck had a partial response, and 13 patients had stable disease according to RECISTv1.1, eight of whom had prolonged periods (12 weeks) of stable disease. -

Veno-Occlusive Disease (VOD) AKA Hepatic Sinusoidal Obstruction Syndrome (SOS) Mira A
Veno-Occlusive Disease (VOD) AKA Hepatic Sinusoidal Obstruction Syndrome (SOS) Mira A. Kohorst, MD MD Anderson Cancer Center Disclosures • No financial disclosures • No discussion of off-label use of medication Roadmap • Pathophysiology • Risk factors • Clinical presentation • Diagnosis • Treatment • Critical care management • Summary PATHOPHYSIOLOGY Injury to the hepatic venous endothelium (sinusoidal endothelial cells and zone 3 hepatocytes) – conditioning chemotherapy, gemtuzumab/inotuzumab, radiation (>30 Gy) Hepatocytes Space of Disse Sinusoid Sinusoidal endothelial cells Richardson PG et al. Expert Opin Drug Saf 2013;12:123–36 Activation of endothelial cells → ↑thrombosis, ↓fibrinolysis, ↑inflammation, ↓cytoskeletal structure → Narrowing of sinusoids Richardson PG et al. Expert Opin Drug Saf 2013;12:123–36 Increased thrombosis and decreased thrombolysis → VOD/SOS Richardson PG et al. Expert Opin Drug Saf 2013;12:123–36 RISK FACTORS Risk Factors Corbacioglu, Jabbour, and Mohty. Biology of Blood and Marrow Transplantation (2019) 25:1271-80. Risk Factors • Pediatric patients have a 2- to 3-fold higher incidence than adults, estimated at 20-30% – Infants 30% (as high as 60%) – Osteopetrosis 60% – Neuroblastoma 30% – Macrophage activating syndromes 30% – Thalassemia 30% Strouse et al. Biology of Blood and Marrow Transplantation (2018). Corbacioglu et al. Expert Rev Hematol (2012). Cesaro et al. Haematologica (2005). CLINICAL PRESENTATION Clinical Presentation Faraci et al. Biology of Blood and Marrow Transplantation. Oct 2018. Clinical Presentation • Weight gain (fluid retention) – first • Can see thrombocytopenia and platelet refractoriness • Hyperbilirubinemia/jaundice, transaminitis – Anicteric in ~30% of pediatric patients • Firm, painful hepatomegaly with ascites • Renal insufficiency (hepatorenal syndrome) in 50% – Need for dialysis in 25% • Multiorgan failure, hepatic encephalopathy, death Corbacioglu et al.