The Grey Zone in Doping
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ephedrine Anyone Used This ? I've Just Bought a Bottle of These Bad Boys & the Wife Has Decided to Google It
Thread: Ephedrine fingersweaver Apr 26, 2007 08:26:29 PM Subject: Ephedrine Anyone used this ? I've just bought a bottle of these bad boys & the wife has decided to google it . As you can imagine she then read me the riot act about side effects etc etc . Do any of you have any genuine experience of ephedrine ? Cheers , Fingers ;\) Wurzeluk Apr 26, 2007 09:17:26 PM Subject: Ephedrine Yes of course it a staple of bodybuilding fat loss .. But is not without side effects , like Steroids it is illegal to supply without prescription in the Uk but not illegal to posess and use personally .. It is safe to use if you are healthy but can cause serious problems if you have high BP , heart problems or are really obese ( yes its a fat burner but only really suitable for fit people with a Bf of 20% or less ) Side effects vary depending on the individual but include jitterness , edginess , headache , heart palpitations etc .. my advice would be start with a small dose to see how you react and ramp up , if you experiance any unpleasant sides then cut back or stop BTW it is much more effective with CAffeine and Aspirin ie the ECA stack you want about 20-30mg Eph 200 mg Caffeine and 15 mg Aspirin a day for best results .. BUT do not expect miracles if a) your Bf is higher than 20% b) your diet is kak Wurz Deleted User Rank: Newbie Apr 26, 2007 09:35:17 PM Subject: Ephedrine I'm interested in trying ephedrine. -

Safety Data Sheet
SAFETY DATA SHEET SECTION 1: PRODUCT IDENTIFICATION PRODUCT NAME DHEA (Prasterone) (Micronized) PRODUCT CODE 0733 SUPPLIER MEDISCA Inc. Tel.: 1.800.932.1039 | Fax.: 1.855.850.5855 661 Route 3, Unit C, Plattsburgh, NY, 12901 3955 W. Mesa Vista Ave., Unit A-10, Las Vegas, NV, 89118 6641 N. Belt Line Road, Suite 130, Irving, TX, 75063 MEDISCA Pharmaceutique Inc. Tel.: 1.800.665.6334 | Fax.: 514.338.1693 4509 Rue Dobrin, St. Laurent, QC, H4R 2L8 21300 Gordon Way, Unit 153/158, Richmond, BC V6W 1M2 MEDISCA Australia PTY LTD Tel.: 1.300.786.392 | Fax.: 61.2.9700.9047 Unit 7, Heritage Business Park 5-9 Ricketty Street, Mascot, NSW 2020 EMERGENCY PHONE CHEMTREC Day or Night Within USA and Canada: 1-800-424-9300 NSW Poisons Information Centre: 131 126 USES Adjuvant; Androgen SECTION 2: HAZARDS IDENTIFICATION GHS CLASSIFICATION Toxic to Reproduction (Category 2) PICTOGRAM SIGNAL WORD Warning HAZARD STATEMENT(S) Reproductive effector, prohormone. Suspected of damaging fertility or the unborn child. May cause harm to breast-fed children. Causes serious eye irritation. Causes skin and respiratory irritation. Very toxic to aquatic life with long lasting effects. AUSTRALIA-ONLY HAZARDS Not Applicable. PRECAUTIONARY STATEMENT(S) Prevention Wash thoroughly after handling. Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Do not breathe dusts or mists. Do not eat, drink or smoke when using this product. Avoid contact during pregnancy/while nursing. Wear protective gloves, protective clothing, eye protection, face protection. Avoid release to the environment. Response IF ON SKIN (HAIR): Wash with plenty of water. -

Vargas KEA, Et Al. Hepatotoxicity Associated with Methylstenbolone and Copyright© Vargas KEA, Et Al
1. Medical Journal of Clinical Trials & Case Studies ISSN: 2578-4838 Hepatotoxicity Associated with Methylstenbolone and Stanozolol Abuse Vargas KEA*, Guaraná TA, Biccas BN, Agoglia LV, Carvalho ACG, Case Report Gismondi R and Esberard EBC Volume 2 Issue 5 Received Date: July 27, 2018 Department of Gastroenterology/Hepatology, Department of Clinical Medicine, and Published Date: September 03, 2018 Department of Pathology, Antônio Pedro University Hospital, Federal Fluminense DOI: 10.23880/mjccs-16000176 University, Rio de Janeiro, Brazil *Corresponding author: Vargas Karen Elizabeth Arce, Department of Gastroenterology/Hepatology, Department of Clinical Medicine, and Department of Pathology, Antônio Pedro University Hospital, Federal Fluminense University, Rio de Janeiro, Ernani do Amaral Peixoto Avenue, 935. Ap.901 / Cep.24020043, Brazil, Tel: 005521981584624; Email: [email protected] Abstract Background & Objectives: Drug hepatotoxicity is a major cause of liver disease. Many drugs are well known to induce liver damage. Some toxic products, like anabolic androgenic steroids, that are pharmaceutical preparations since they contain pharmaceutically active substance, are available as nutritional supplements. Many patients are used to consume these like dietary stuff. Methods: We introduce a case series of two patients who developed hepatic damage after the consumption of anabolic- androgenic steroids, accompanied by a detailed bibliographic research on this topic. Results: We present two young men who developed significant liver damage, both with hyperbilirubinemia pattern after consumption of anabolic-androgenic steroids. This was associated with considerable morbidity, although both recovered without liver transplantation. The two anabolic-androgenic steroids were being marketed as dietary supplements. Conclusions: Although not well controlled substances in Brazil, anabolic-androgenic steroids are cause of severe hepatotoxicity. -

How to Gain up to 15 Lbs. of Muscle in 6 Weeks Using Potent "Homemade" Supplement Stacks and Mixes
How to gain up to 15 lbs. of muscle in 6 weeks using potent "homemade" supplement stacks and mixes By Lee Hayward & Bryan Kernan "Cutting-edge E-book Reveals Secrets to Turning Ordinary Supplements Into "Turbo-Charged" Anabolic Compounds" © 2001 Lee Hayward Enterprises 1 Bodybuilding Supplement Secrets Revealed: How to gain up to 15 lbs. of muscle in 6 weeks using potent "homemade" supplement stacks and mixes By Lee Hayward & Bryan Kernan © 2002 Lee Hayward Enterprises All Rights Reserved. International Copyright Bodybuilding Supplement Secrets Revealed © 2001 Lee Hayward Enterprises 2 Notice The information presented is not intended for the treatment or prevention of disease, nor a substitute for medical treatment, nor as an alternative to medical advice. This publication is presented for information purposes, to increase the public knowledge of developments in the field of supplements. The program outlined herein should not be adopted without a consultation with your health professional. Use of the information provided is at the sole choice and risk of the reader. Bodybuilding Supplement Secrets Revealed © 2001 Lee Hayward Enterprises 3 Dear Friend, I have been involved with bodybuilding for the past 10 years and I have seen fads, trends, and scams come and go. I've literally spent thousands of dollars on supplements, magazines, training systems, diets and weights in my effort to find the most powerful way to put muscle on fast. I've also seen and studied how the supplement industry operates. Both as a customer buying up the latest craze, and as an "insider" with my own company. I must issue you a warning here. -

Records of Pharmaceutical and Biomedical Sciences
REVIEW ARTICLE RECORDS OF PHARMACEUTICAL AND BIOMEDICAL SCIENCES Effect of Exogenous Anabolic Androgenic Steroids on Testosterone/ Epitestosterone Ratio and its Application on Athlete Biological Passport in Egypt Hanem A. Khalil a, Dina M. Abo-Elmatty b, Rosa V. Alemany c, Noha M. Mesbah b a Egyptian Anti-Doping Organization, Cairo, Egypt. b Faculty of Pharmacy, Department of Biochemistry Suez Canal University, Ismailia, Egypt. C Catalonian Anti-Doping Laboratory of Fundacio IMIM, Barcelona, Spain. Abstract Received on: 01.09. 2018 Using the Anabolic Androgenic Steroid (AAS) agents is evident not only Revised on: 21. 10. 2018 within the competitive senior and junior athletes, but also in non-sporting contexts by individuals seeking to „improve‟ their physique. No accurate data Accepted on: 01. 11. 2018 is available for the prevalence of AAS misuse among athletes. Studies suggest that it may be 1–5% of the population; with the prevalence being higher in males. Many studies documented side effects and health hazards with the misuse of anabolic steroids, where these were accused as a cause of Correspondence Author: deaths among athletes. Intake of exogenous anabolic steroids disturbed the Testosterone / Epitestosterone (T/E) ratio causing its evaluation above the Tel:+201270206648. normal level. This review outlines the anabolic steroids, its side effects and E-mail address: health impacts in both the sporting and physique development contexts. It also provides a brief review of the history of AAS as doping agents and [email protected] athlete biological passport. Conclusion: Doping among athletes is a widespread public health and social problem. Many studies have shown that both short- and long-term health complications have consequences and dependencies. -

The Metabolism of Anabolic Agents in the Racing Greyhound
The Metabolism of Anabolic Agents In the Racing Greyhound A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy by Mr. Keith Robert Williams, B.Sc. July 1999 Department of Forensic Medicine & Science University of Glasgow Copyright © 1999 by Keith R. Williams. All rights reserved. No part o f this thesis may be reproduced in any forms or by any means without the written permission o f the author. I ProQuest Number: 13833925 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13833925 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 GLASGOW UNIVERSITY LIBRARY 111-X (coK To my parents for all their help, support and encouragement i Table of Contents i List of Figures V List of Tables VIII Summary IX Chapter 1: Drugs in Sport ...............................................................................................................................1 Introduction ................................................................................................................................................. -

Reproductive DHEA
Reproductive DHEA Analyte Information - 1 - DHEA Introduction DHEA (dehydroepiandrosterone), together with other important steroid hormones such as testosterone, DHT (dihydrotestosterone) and androstenedione, belongs to the group of androgens. Androgens are a group of C19 steroids that stimulate or control the development and maintenance of male characteristics. This includes the activity of the male sex organs and the development of secondary sex characteristics. Androgens are also precursors of all estrogens, the female sex hormones. DHEA (dehydroepiandrosterone) is the aromatic C19-steroid composed of a 10,13-dimethyl, 3-hydroxy group and 17-ketone. Its chemical name is 3β-hydroxy-5-androsten-17-one, its summary formula is C19H28O2, and its molecular weight (Mr) is 288.4 Da. The structural formulas of DHEA and related androgens are shown in Fig.1 Fig.1: Structural formulas of the most important androgens DHEA Androstenedione Testosterone Dihydrotestosterone There are more than 40 other names used for DHEA, including: (+)-Dehydroisoandrosterone; (3beta, 16alpha)-3,16-dihydroxy-androst-5-en- 17-one; 5,6-Dehydroisoandrosterone; 17-Chetovis, 17-Hormoforin, Andrestenol, Diandron, Prasterone and so on. As DHEA is very closely connected with its sulfate form DHEA-S, both hormones are mentioned together in the following text. Biosynthesis DHEA is the steroid hormone belonging to the weak androgens. DHEA and DHEA-S are the major C19 steroids produced from cholesterol by the zona reticularis of the adrenal cortex (Fig.2). DHEA is also produced in small quantities in the gonads (testis and ovary3,8,14), in adipose tissue and in the brain. From this point of view DHEA belongs to the neurosteroids22. -

Steroids and Other Appearance and Performance Enhancing Drugs (Apeds) Research Report
Research Report Revised Febrero 2018 Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Table of Contents Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Introduction What are the different types of APEDs? What is the history of anabolic steroid use? Who uses anabolic steroids? Why are anabolic steroids misused? How are anabolic steroids used? What are the side effects of anabolic steroid misuse? How does anabolic steroid misuse affect behavior? What are the risks of anabolic steroid use in teens? How do anabolic steroids work in the brain? Are anabolic steroids addictive? How are anabolic steroids tested in athletes? What can be done to prevent steroid misuse? What treatments are effective for anabolic steroid misuse? Where can I get further information about steroids? References Page 1 Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Esta publicación está disponible para su uso y puede ser reproducida, en su totalidad, sin pedir autorización al NIDA. Se agradece la citación de la fuente, de la siguiente manera: Fuente: Instituto Nacional sobre el Abuso de Drogas; Institutos Nacionales de la Salud; Departamento de Salud y Servicios Humanos de los Estados Unidos. Introduction Appearance and performance enhancing drugs (APEDs) are most often used by males to improve appearance by building muscle mass or to enhance athletic performance. Although they may directly and indirectly have effects on a user’s mood, they do not produce a euphoric high, which makes APEDs distinct from other drugs such as cocaine, heroin, and marijuana. However, users may develop a substance use disorder, defined as continued use despite adverse consequences. -

Michigan Department of Community Health
Michigan Department 0f Community Health, Office of Drug Control Policy The Michigan Association of Community Mental Health March 27, 2009 NationalNational TrendsTrends andand DeterrentDeterrent StrategiesStrategies ForFor PrescriptionPrescription andand OTCOTC DrugDrug AbuseAbuse Joseph Rannazzisi Deputy Assistant Administrator Office of Diversion Control Drug Enforcement Administration IntroductionIntroduction BackgroundBackground andand StatisticsStatistics RegulatoryRegulatory ControlControl MethodsMethods ofof DiversionDiversion InternetInternet DiversionDiversion CommonlyCommonly DivertedDiverted PharmaceuticalsPharmaceuticals Steroids/Steroids/hGHhGH DietaryDietary SupplementsSupplements SalviaSalvia DivinorumDivinorum TheThe 19601960’’ss Marijuana Seconal LSD Dexedrine Meprobamate TheThe 19701970’’ss Heroin T’s and Blues 4’s and Doors (Talwin and Pyrabenzamine) TheThe 19801980’’ss Tylenol w/Codeine and Doriden Hydromorphone Cocaine TheThe 19901990’’ss Oxycodone Methamphetamine 20002000 Hydrocodone Ketamine Alprazolam Flunitrazapam MDMA (Rohypnol) Scope and Extent of Problem 2004 2007 0.30.3 millionmillion 0.35 million SedativesSedatives 1.21.2 millionmillion 1.11.1 millionmillion StimulantsStimulants 1.61.6 millionmillion 1.81.8 millionmillion Anti-Anxiety Medication 4.44.4 millionmillion 5.25.2 millionmillion NarcoticNarcotic PainPain RelieversRelievers Source: 2004 and 2007 National Survey on Drug Use and Health TeensTeens andand TheirTheir AttitudesAttitudes 1 in 5 teens report abusing Rx medications to get high 2 in -

Pharmacopoeial Reference Standards and Their Current Lot Numbers EP, EPISA, ICRS, BP July 2019
Pharmacopoeial reference standards and their current lot numbers EP, EPISA, ICRS, BP July 2019 Mikromol Follow LGC on LinkedIn Dear user, This catalogue includes all pharmacopoeial reference materials from EP, EPISA, ICRS and BP available at LGC, as well as their current lot numbers. We update the catalogue on a monthly basis so that you can use the lot numbers to easily track the expiry dates of your stocked reference materials. We took the lot information from actual data from pharmacopoeias, correct at the time of production. We retained all spelling and information as provided by the pharmacopoeias. The list was compiled with caution, however, errors in this list may be possible, and LGC is not responsible for any consequences as a result of these errors, may it be errors in the original Pharmacopoeial data, or from LGC during compilation of this list. For any questions or orders please contact your local LGC office. You will find all contact details on the last page of this list. Alternatively, you can browse and buy pharmaceutical reference materials online at lgcstandards.com/mikromol. Stay connected via LinkedIn (LGC Mikromol) or Twitter @LGCMikromol. Kind regards Your LGC Standards Team LGC, Queens Road, Teddington, Middlesex, TW11 0LY, United Kingdom Tel: +44 (0)20 8943 8480 Fax: +44 (0)20 8943 7554 E-mail: [email protected] European Pharmacopoeia (EP) Available Current Unit Cat. No. Name What is new? since Batch No. Quantity EPY0001552 Abacavir for peak identification 1 10 mg EPY0001551 Abacavir for system suitability 1 -
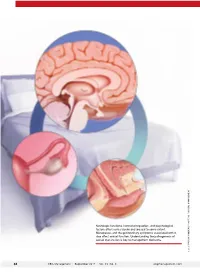
Neurologic Functions, Hormonal Regulation, and Psychological Factors Affect Sexual Desire and Arousal to Some Extent
Neurologic functions, hormonal regulation, and psychological factors affect sexual desire and arousal to some extent. Menopause, and the genitourinary symptoms associated with it, also affect sexual function. Understanding the pathogenesis of sexual dysfunction is key to management decisions. ILLUSTRATION: KIMBERLY MARTENS FOR OBG MANAGEMENT MARTENS KIMBERLY ILLUSTRATION: 34 OBG Management | September 2017 | Vol. 29 No. 9 obgmanagement.com UPDATE FEMALE SEXUAL DYSFUNCTION New and emerging treatment options hold promise for improving outcomes in this undertreated disorder ❯❯ Barbara S. Levy, MD Dr. Levy is Vice President for Health Policy at the American College of Obstetricians and Gynecologists, Washington, DC. The author reports no financial relationships relevant to this article. exual function is a complex, multifac- chronic conditions such as diabetes and S eted process mediated by neurologic back pain.4 functions, hormonal regulation, and psy- Understanding the pathogenesis of chological factors. What could possibly female sexual dysfunction helps to guide go wrong? our approach to its management. Indeed, IN THIS As it turns out, quite a lot. Female sex- increased understanding of its pathology has ARTICLE ual dysfunction is a common, vastly under- helped to usher in new and emerging treat- treated sexual health problem that can have ment options, as well as a personalized, bio- How hormones, wide-reaching effects on a woman’s life. psychosocial approach to its management. experience, These effects may include impaired body In this Update, I discuss the interplay of and behavior affect image, self-confidence, and self-worth. Sex- physiologic and psychological factors that the brain ual dysfunction also can contribute to rela- affect female sexual function as well as the page 36 tionship dissatisfaction and leave one feeling latest options for its management. -

LEGISLATIVE ASSEMBLY Question on Notice
LEGISLATIVE ASSEMBLY Question On Notice Thursday, 15 February 2018 2560. Ms M. M Quirk to the Minister for Police; I refer to the commencement of operation of the Road Traffic Amendment Act 2016 in March 2017 which introduced compulsory blood or urine samples to be taken from drivers involved in serious crashes, and I ask: (a) since March 2017 how many such samples have been taken; (b) for what specific substances are those blood or urine samples tested; (c) what are the results of those tests to date; and (d) what percentage of drivers have been found to have ingested more than one substance capable of impairing driving skills? Answer (a) The compulsory taking of blood from all drivers involved in serious crashes commenced on 10 March 2017. Between 10 March 2017 and 22 February 2018 (inclusive) a total of 398 blood test samples have been collected under the provision of the Road Traffic Amendment Act 2016. There have been no urine tests collected. (b) Please see attached table for a list of substances in blood sample that are identifiable in ChemCentre toxicology analysis (Paper Number). (c) Of the 398 blood samples collected, 48 are pending results of ChemCentre analysis. Of the 350 analysed, 259 samples had a specific substance(s) detected and 91 samples had no specific substance detected. d) Of the 259 samples with specific substance(s) detected, 92% were found to have multiple substances (more than one). Detectable Substances in Blood Samples capable of identification by the ChemCentre WA. ACETALDEHYDE AMITRIPTYLINE/NORTRIPTYLINE