Radiant Pharma Form I Annexure
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

GA-10.03 CHITTOOR, KOLAR and VELLORE DISTRICTS.Pdf
77°50'0"E 78°0'0"E 78°10'0"E 78°20'0"E 78°30'0"E 78°40'0"E 78°50'0"E 79°0'0"E 79°10'0"E 79°20'0"E 79°30'0"E 79°40'0"E 79°50'0"E 80°0'0"E GEOGRAPHICAL AREA CHITTOOR, KOLAR AND N N " " VELLORE DISTRICTS 0 0 ' ' 0 0 ° ° 4 ± 4 1 1 Peddamandyam ! CA-03 CA-05 KEY MAP PEDDAMANDYAM MULAKALACHERUVU ! Kalicherla N CA-52 N " CA-11 " 0 Sompalle CA-04 CA-06 CA-60 0 ' ! SRIKALAHASTI ' 0 Veligallu KAMBHAMVARIPALLE 0 5 THAMBALLAPA! LLI ! GURRAMKONDA ! THOTTAMBEDU 5 ° ° 3 Thamballapalle Kalakada Kambhamvaripalle CA-21 3 1 Mulakalacheruvu 1 ! ! Á! CA-10 YERRAVARIPALEM 565 ANDHRA Gurramkonda ! ¤£ CA-02 ! Pedda Kannali PRADESH Kosuvaripalle KALAKADA CA-20 Bodevandlapalle Á! ! PEDDATHIPPASAMUDRAM ! Gundloor PILERU KARNATAKA ! CA-51 CA-53 (! Á! CA-40 Á! Á! Pattamvandlapalle Burakayalakota RENIGUNTA Srikalahasti ! ! TIRUPATI Á! YERPEDU Peddathippasamudram Rangasamudram ! ! ! Maddin!ayanipalCle H MudIivedu T T O O R CA-22 URBAN Á! Á ! ¤£31 CA-12 ! Karakambadi (Rural) ! ROMPICHERLA Á ! ! N Á N " Thummarakunta CA-07 KALIKIRI (! Tirumala CA-61 " 0 0 ' ! ' CA-09 Rompicherla ! Á 0 B.Kothakota KURÁ!ABALAKOTA ! Mangalam 0 4 ! CA-01 Á Chinnagotti Gallu ! BN 4 ° 71 ( ° ! VALMIKIPURAM Kalikiri ¤£ (! ! CA-39 3 Pileru 3 ! ! ! Renigunta 1 B Kurabalakota Á! ! KHANDRIGA 1 Thettu ! Á Akkarampalle (! TA M I L N A D U ChinnathippasamudÁ!ram Á!Chintaparthi CHINNAGOTTIGALLU (! ! Á! KOTHAKOTA ! ! Á! Kalikirireddivari Palle ! Doddipalle ! Á! Á Vikruthamala Badikayalapalle ! Angallu ! (! Á ! Kothavaripalle Á! CA-4(!1 ! Valmikipuram Á! Cherlopalle (! Varadaiahpalem Gattu ! ! ! Daminedu -

Andhra Pradesh Public Disclosure Authorized October, 2016
SFG2380 REV ` Environment and Public Disclosure Authorized Social Management Public Disclosure Authorized Framework for Power Transmission and Public Disclosure Authorized Distribution Projects Andhra Pradesh Public Disclosure Authorized October, 2016 Page 1 Table of Contents ACRONYMS AND ABBREVIATIONS 4 1. EXECUTIVE SUMMARY 6 2. INTRODUCTION 34 2.1 Project Context 34 A. Proposed Investments for APTRANSCO 34 B. Proposed Investments for APSPDCL and APEPDCL 36 2.2 Purpose of ESMF 38 3. SOCIO-ECONOMIC PROFILE OF STATE 39 4. ENVIRONMENT PROFILE OF STATE 42 5. APPROACH AND METHODOLOGY 50 6. STAKEHOLDER ANALYSIS 53 7. SOCIAL IMPACT & MITIGATION METHODS 64 7.1 Social Impacts 64 7.2 Policy and Legal Framework-Social 69 7.3 Management & Mitigation Methods: Social 88 A. Loss of Land, Resettlement and Rehabilitation 88 B. Change in Land Use or Restrictions to Land Use 102 C. Community Health and Safety 103 D. Impact on workers/employees health and safety 105 E. Impact on vulnerable population 106 F. Cultural Heritage 108 G. Interference with communication channels 108 8. ENVIORNMENTAL IMPACTS & MITIGATION METHODS 109 8.1 Environmental Screening and Analysis of Alternatives 109 8.2 Environmental Impacts and mitigation measures 110 8.3 Policy and Legal Framework 130 9. INSTITUTIONAL ARRANGEMENTS 137 9.1 APTRANSCO 137 9.2 APSPDCL/APEPDCL 145 10. GRIEVANCE REDRESSAL MECHANISM 147 Page 2 11. MONITORING PLAN 150 12. TRAINING AND CAPACITY BUILDING 158 13. COST AND BUDGET 159 14. CONSULTATIONS AND DISCLOUSRE 162 ANNEXURE 1 – DEFINITIONS 167 ANNEXURE 2 – -

List-Of-TO-STO-20200707191409.Pdf
Annual Review Report for the year 2018-19 Annexure 1.1 List of DTOs/ATOs/STOs in Andhra Pradesh (As referred to in para 1.1) Srikakulam District Vizianagaram District 1 DTO, Srikakulam 1 DTO, Vizianagaram 2 STO, Narasannapeta 2 STO, Bobbili 3 STO, Palakonda 3 STO, Gajapathinagaram 4 STO, Palasa 4 STO, Parvathipuram 5 STO, Ponduru 5 STO, Salur 6 STO, Rajam 6 STO, Srungavarapukota 7 STO, Sompeta 7 STO, Bhogapuram 8 STO, Tekkali 8 STO, Cheepurupalli 9 STO, Amudalavalasa 9 STO, Kothavalasa 10 STO, Itchapuram 10 STO, Kurupam 11 STO, Kotabommali 11 STO, Nellimarla 12 STO, Hiramandalam at Kothur 12 STO, Badangi at Therlam 13 STO, Pathapatnam 13 STO, Vizianagaram 14 STO, Srikakulam East Godavari District 15 STO, Ranasthalam 1 DTO, East Godavari Visakhapatnam District 2 STO, Alamuru 1 DTO, Visakhapatnam 3 STO, Amalapuram 2 STO, Anakapallli (E) 4 STO, Kakinada 3 STO, Bheemunipatnam 5 STO, Kothapeta 4 STO, Chodavaram 6 STO, Peddapuram 5 STO, Elamanchili 7 DTO, Rajahmundry 6 STO, Narsipatnam 8 STO, R.C.Puram 7 STO, Paderu 9 STO, Rampachodavaram 8 STO, Visakhapatnam 10 STO, Rayavaram 9 STO, Anakapalli(W) 11 STO, Razole 10 STO, Araku 12 STO, Addateegala 11 STO, Chintapalli 13 STO, Mummidivaram 12 STO, Kota Uratla 14 STO, Pithapuram 13 STO, Madugula 15 STO, Prathipadu 14 STO, Nakkapalli at Payakaraopeta 16 STO, Tuni West Godavari District 17 STO, Jaggampeta 1 DTO, West Godavari 18 STO, Korukonda 2 STO, Bhimavaram 19 STO, Anaparthy 3 STO, Chintalapudi 20 STO, Chintoor 4 STO, Gopalapuram Prakasam District 5 STO, Kovvur 1 ATO, Kandukuru 6 STO, Narasapuram -

Premises Application for PALACE ROAD BRANCH- KUPPAM Br Page 1 out of 12
THE REGIONAL MANAGER, STATE BANK OF INDIA REGIONAL BUSINESS OFFICE IV, TIRUPATHI RURAL, AO, TIRUPATI – 517501 Phone no : 0877 – 2237250 Fax no : 0877 – 2237259 ___________________________________________________________________ Website: www.sbhyd.com/Tenders REQUIREMENT OF ALTERNTE PREMISES TO OUR ” PALACE ROAD BRANCH-, KUPPAM ”, AT CHITTOOR DIST. ANDHRA PRADESH Our Bank requires Premises on lease basis for shifting our existing PALACE ROAD BRANCH-KUPPAM in to an alternate premises at KUPPAM, CHITTOOR DIST. ANDHRA PRADESH, having rentable area of approximately 3500-4000 sq.ft in KUPPAM TOWN, CHITTOOR DIST. ANDHRA PRADESH. Tender forms and complete details can be obtained from Regional Office at the above address or down- load from our Bank's website at www.sbi.co.in/eprocurements Willing Landlords/Owners of the premises may submit the completely filled tender documents in two separate sealed envelopes, superscribed "Technical Bid PALACE ROAD BRANCH " and "Financial Bid PALACE ROAD BRANCH -KUPPAM ", to the REGIONAL MANAGER, State Bank of India, Region – IV, ADMINIS- TRATIVE OFFICE, 4TH FLOOR, KALANJALI BUILDING, RENIGUNTA RAOD, TIRUPATI- so as to reach latest by 3.00 PM on 30-11-2017. Tenders will be opened at 4.00 PM on 30-11-2017 and for further details please contact the under signed. Tenders may be downloaded from the bank’s website(www.sbi.co.in/eprocurements) and the same to be submitted by depositing in theTender box provided in the office of REGIONAL MANAGER, REGION IV, RBO, TIRUPATI RURAL, 4TH FLOOR, KALANJALI BUILDING, RENIGUNTA RAOD, TIRUPATI -517 501. Tenders proform should not be altered/ modified/changed as otherwise the your tenders stands rejected. -

The Kuppam Kalanjia Samakhya Experience
Restoring Trust – The Kuppam Kalanjia Samakhya Experience - J.Dharmendra kumar, Managing Director, Kuppam kalanjia Samakhya Background of the location Chittoor district of Andhra Pradesh in Rayalaseema region was characterized by backwardness and poor performance in basic social indicators. Kuppam is one of the mandal in Chittor district, which is in the extreme corner of AP, surrounded by Tamilnadu on eastern and southern sides and on west by Karnataka. In order to strengthen the existing DWCRA groups and regularize the systems DRDA has approached DHAN Foundation and made an agreement in the month of Nov. 99. The main objective of this project was graduating the existing DWCRA groups in to savings and credit groups and promotion of mandal level federation. DHAN Foundation would act as an implementing agency and has agreed to implement the project. DRDA, chittoor would provide necessary guidance and promotional cost for implementing the project in installment on half-yearly basis. Dhan entered in the month of January 2000 with the objective of initiating a process of demonstrating successful establishment of savings and credit groups which will emerge in to a self managed federation to provide financial services to the poor. At present Kuppam Kalanjia Samakhya is working with 253 groups in 104 villages with 3536 and 10 restructured cluster development associations. The starting scenario Kuppam mandal is located in a very remote and backward area in Andhra Pradesh. The socio-economic status of the people, especially women is very low. Due to extreme poverty, people frequently required small loans. “However banks are unwilling to entertain these amounts. -
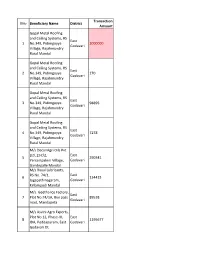
List of Units Uploaded in Finance Dept Portal for Release
Transaction SlNo Beneficiary Name District Amount Gopal Metal Roofing and Ceiling Systems, RS East 1 No.349, Pidimgoyya 2000000 Godavari Village, Rajahmundry Rural Mandal Gopal Metal Roofing and Ceiling Systems, RS East 2 No.349, Pidimgoyya 270 Godavari Village, Rajahmundry Rural Mandal Gopal Metal Roofing and Ceiling Systems, RS East 3 No.349, Pidimgoyya 98895 Godavari Village, Rajahmundry Rural Mandal Gopal Metal Roofing and Ceiling Systems, RS East 4 No.349, Pidimgoyya 7278 Godavari Village, Rajahmundry Rural Mandal M/s DeconAgri Oils Pvt Ltd, 257/2, East 5 290341 Yerrampalem Village, Godavari Gandepalle Mandal M/s Royal Lubricants, RS No. 74/1, East 6 134415 Jagapathinagaram, Godavari Kirlampudi Mandal M/s. Geetha Ice Factory, East 7 Plot No.74/1A, Bye pass 89538 Godavari road, Mandapeta M/s Asvini Agro Exports, Plot No 12, Phase-III, East 8 1195677 IDA, Peddapuram, East Godavari Godavari Dt M/s Sri Padma Refractory Monolithics, East 9 RS No 99/1, 164412 Godavari Neeladriraopeta, Gandepalle Mandal M/s Sri Padma Refractory Monolithics, East 10 RS No 99/1, 60427 Godavari Neeladriraopeta, Gandepalle Mandal M/s Sri Vajra Electro Porcelains Pvt Ltd, RS East 11 No 164, VT Puram Road, 62157 Godavari Peddapruam, East Godavari Dt M/s. Auro Energy, East 12 Balabadrapuram village, 181877 Godavari Biccavolu(M) M/s. Sri Hari Bio Fuels, R.S.No.144/2, East 13 9453 Malapadu, Godavari Ramachandrapuram M/s. Sri Hari Bio Fuels, R.S.No.144/2, East 14 31719 Malapadu, Godavari Ramachandrapuram M/s. Sri Buvanas Enterprises, Plot No.8, East 15 510983 phase-III, IDA, Godavari Peddapuram M/s. -

How to Reach Iit Tirupati Academic Building, Boys Hostel and Girls Hostel
HOW TO REACH IIT TIRUPATI ACADEMIC BUILDING, BOYS HOSTEL AND GIRLS HOSTEL S. No. From To Distance Mode (fare is Route approx.) 1 Tirupati 8 Kms By Share Via Ramanuja Railway Auto – Circle, Auto Nagar, Station/Bus Rs.20/- and pass Krishna Theja Stand Individual Educational Auto Institutions, take Rs.100/- right turn before ECIL and follow direction boards 2 Renigunta Rly Academic 5 Kms By Auto Via Renigunta Fly Station Building (Rs.50/-) Over, ECIL, take left turn after ECIL and follow direction boards 3 Airport 12 Kms By pre-paid Via Renigunta Fly Taxi Over, ECIL, take left (Rs.650/-) turn after ECIL and follow direction boards. 4 Tirupati Rly 28 Kms By Bus upto Via Ramanuja Stn/Bus Stand Yerpedu Circle, Auto Nagar, Junction and Krishna Theja by Auto from Educational Yerpedu Institutions, Junction to Renigunta Fly Over, Permanent Renigunta Junction, Campus Yerpedu Junction, Boys Hostel Yerpedu – (All the Venkatagiri Road, years) Cross Railway Gate, Mandal Office, Permanent Campus 5 Renigunta Rly 18 By Bus upto Renigunta Junction, Station Yerpedu Yerpedu Junction, Junction and Yerpedu – by Auto from Venkatagiri Road, Yerpedu Cross Railway Gate, Junction to Mandal Office, Permanent Permanent campus Campus (OR) Auto (Rs.150/-) 6 Airport 12 By pre-paid Airport, Yerpedu Taxi Rs.650/- Junction, Yerpedu – Venkatagiri Road, Cross Railway Gate, Mandal Office Permanent Campus 7 Tirupati Rly 8 Kms By Auto Via Ramanuja Station/ Bus Circle, Auto Nagar, Stand after Krishna Theja Educational Institutions take right turn before ECIL, go past Sai Baba Temple and Girls Hostel, following direction Lakshminagar boards to Girls colony (Next Hostel. -

Analysis of Ground Water Potential in Chandragiri Mandal, Chittoor District, Andhra Pradesh
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2013, 4(4):255-265 ISSN: 0976-8610 CODEN (USA): AASRFC Analysis of ground water potential in Chandragiri Mandal, Chittoor District, Andhra Pradesh Bhupal. K and Reddi Bhaskara Reddy. M Dept. of Geography, Sri Venkateswara University, Tirupati _____________________________________________________________________________________________ ABSTRACT Ground water prospects of any area depend on its geological structure, geomorphic features and their hydrological characters. Identification and mapping of these elements is thus imperative for ground water exploration and optimal management of this precious resource. In the present paper ground water potentiality in Chandragiri mandal, Chittoor district, Andhra Pradesh has been evaluated by analyzing the hydro geomorphic parameters using Remote sensing Techniques. Satellite image and Topographical map have been used to prepare the required thematic maps like geology, lineaments, geomorphology, surface water bodies and drainage. These maps have been integrated in GIS environment to demarcate the hydro geomorphic units. Nine hydro geomorphic units viz. Flood plain, Moderately Weathered pedi plain, Shallow Weathered Pedi plain, Residual hill, Denudation hill, Structural hill, Inselberg, Pediment and Bajada have been derived from the integrated map. Ground water potentiality has been qualitatively assessed by analyzing the derived hydro geomorphic units after considering the field information. Key words : Ground water potential, Geomorphology, Lineaments, Pediplains, Pediments, Inselburg _____________________________________________________________________________________________ INTRODUCTION Rapid growth of population has projected the demand for food production and opened new ways to improve the utilization of surface and sub-surface water resources recently in a systematic and in a scientific way. The excavation at Mohenjo-Daro have related brick-lined dug wells existing as early as 3000 B.C. -

Data Base of Chittoor District
DATA BASE OF CHITTOOR DISTRICT. The district is categorized under Southern Agro Climatic Zone of Andhra Pradesh based on soil type, rainfall and altitude. There are 66 mandals, 1540 revenue villages and 1394 Panchayats in the district. In dry farming tracts of the zone groundnut is the main crop where as under tanks, wells and bore wells double cropping is practiced with Rice. After Groundnut and Paddy, Sugarcane occupies 3rd place in Chittoor District. At present year, the area under maize and Sunflower is increasing gradually in the district. Information is being collected regularly pertaining to Area, Production and Productivity of Agriculture, Horticulture, Sericulture, Animal husbandry and other related disciplines, updated and computerized systematically CHITTOOR DISTRICT Agricultural lands of the district comprise Red Soils - 57% Sandy loams - 34% Mixed Soils - 9% LAND UTILIZATION PATTERN IN THE DISTRICT (Area in ha) S. Particulars Area No. 1. Forest 4,51,345 2. Barren & Uncultivable land 1,64,265 3. Land Put to Non-Agril. Uses 1,57,000 4. Permanent Pastures & Other grazing lands 36,521 5. Miscellaneous tree crops & Groves not included in net area sown. 25,173 6. Cultivable waste 39,512 7. Other fallow lands 1,26,287 8. Current fallows 1,61,759 9. Net area sown 3,55,674 10. Total Geographical area 14,98,778 11. Total cropped area 4,08,000 12. Area sown more than once 36,283 CHITTOOR DISTRICT FARMING SITUATIONS S. No Farming Situation Total No.of Area (HA) Mandals 1. Medium Irrigation (Canal) Red Soils 15,216 14 2. Minor Irrigation (Tanks) Red Soils 42,368 61 3. -
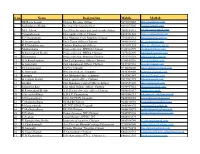
S.No Name Designation Mobile Mail Id
S.no Name Designation Mobile Mail id 1 SK.Razia begum District Revenue Officer 9491077003 [email protected] 2 kodhndarami Reddy Revenue Divisional officer 9491077005 [email protected] 3 M.A. Jaleen Asst.Director.surey and land records.chittor 9866169511 [email protected] 4 Umamaheswar Dist.supply Officer,Chittoor 8008301423 [email protected] 5 E.N.Jayaramulu Dist.Manager,Civil Supplies,Chittoor 7702003533 [email protected] 6 G.Sreenivasulu Divl..Forest Officer,Chittoor 9440810136 [email protected] 7 K.L.Prabhakar rao District Panchayath Officer 9491071325 [email protected] 8 Madhavilatha Project director ,DWMA,Chittoor 9100966779 [email protected] 9 B.Raviprakash Reddy Project director ,DRDA,Chittoor 7675854309 [email protected] 10 Dhananjaya Project director ,Housing,Chittoor 7093930110 [email protected] 11 G.A.Ravichandran Dist.Co-Operative Officer,Chittoor 9100109216 [email protected] 12 K.Samuyelu Dist.Educational Officer,Chittoor 9849909110 [email protected] 13 P.Chandramouli DVEO,Tirupathi 9440816009 [email protected] 14 K.Munnaiah RIO.Intermideate,Tirupathi 9848309000 [email protected] 15 Lavanya Dist.Malariya Officer,chittoor 9849902383 [email protected] 16 G.venkata Prasad Dist.Leprocy officer,Chittoor 9819902375 [email protected] 17 surekha Dist.Blindness control Officer,chittoor 8008553649 [email protected] 18 M.Eswara Rao Dist.tribal welfare officer, Chittoor 9490957021 [email protected] 19 B.Raviprakash Reddy A.D.Disabled welfare officer,Chittoor 9000013617 addwctr@gmail. 20 S.Sreenivaskumar E.D,S.C.Corporation -
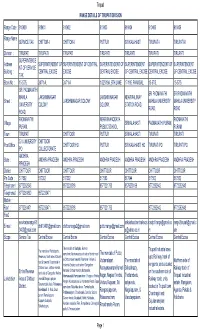
Div and Range Address
Tirupati RANGE DETAILS OF TIRUPATI DIVISION Range Code : 910400 910401 910402 910403 910404 910405 910406 Range Name SERVICE TAX CHITTOR-I CHITTOR-II PUTTUR SRI KALAHASTI TIRUPATI-I TIRUPATI-II : Division : TIRUPATI TIRUPATI TIRUPATI TIRUPATI TIRUPATI TIRUPATI TIRUPATI SUPERINTENDE Address SUPERINTENDENT OF SUPERINTENDENT OF CENTRAL SUPERINTENDENT OF SUPERINTENDENT SUPERINTENDENT OF SUPERINTENDENT NT OF SERVICE Building : CENTRAL EXCISE EXCISE CENTRAL EXCISE OF CENTRAL EXCISE CENTRAL EXCISE OF CENTRAL EXCISE TAX Block No : 15-57/5, 24/71-A, 24/71-A 1/325/19A, 6TH LANE 17-195, PANAGAL, 15-57/5, 15-57/5, SRI PADMAVATHI SRI PADMAVATHI SRI PADMAVATHI MAHILA LAKSHMINAGAR LAKSHMI NAGAR NEAR RALIWAY Street : LAKSHMINAGAR COLONY MAHILA UNIVERSITY MAHILA UNIVERSITY UNIVERSITY COLONY, COLONY, STATION ROAD, ROAD, ROAD ROAD, PADMAVATHI NEAR BHANODAYA PADMAVATHI Village : SRIKALAHASTI PADMAVATHI PURAM, PURAM, PUBLIC SCHOOL, PURAM, Town : TIRUPATI CHITTOOR PUTTUR SRIKALAHASTI TIRUPATI TIRUPATI S.V.UNIVERSITY CHITTOOR Post Office : CHITTOOR HO PUTTUR SRI KALAHASTI HO TIRUPATI PO TIRUPATI PO PO COLLECTORATE ANDHRA State : ANDHRA PRADESH ANDHRA PRADESH ANDHRA PRADESH ANDHRA PRADESH ANDHRA PRADESH ANDHRA PRADESH PRADESH District : CHITTOOR CHITTOOR CHITTOOR CHITTOOR CHITTOOR CHITTOOR CHITTOOR Pin Code : 517502 517002 517002 517583 517644 517502 517502 Telephone1 : 8772262546 8572220518 8577221753 8578230199 8772262642 8772252643 Telephone2 : 8772261830 8572233471 Mobile : Fax1 : 8772261471 8572233471 8572220518 8577221753 8772262642 8772262643 Fax2 : servicetaxrange91 -

Water Quality Monitoring on Tirumala and Tirupati, Andhra Pradesh, India
Available online a t www.derpharmachemica.com Scholars Research Library Der Pharma Chemica, 2012, 4 (3):1074-1079 (http://derpharmachemica.com/archive.html) ISSN 0975-413X CODEN (USA): PCHHAX Water Quality monitoring on Tirumala and Tirupati, Andhra Pradesh, India K. Raju and T. Damodharam* Dept. of Environmental Sciences, SVU College of Sciences, S.V.University, Tirupati-517502 , A.P. India. ______________________________________________________________________________ ABSTRACT An attempt has been made to evaluate the water quality of supplemented and ground water in Tirumala and Tirupati, Chittoor District, Andhra Pradesh, India. The Tirumala and Tirupati are the most popular pilligramage and education areas in Andhra Pradesh. Twelve areas of Tirumala and Tirupati have been selected, where the peoples are used supplemented and groundwater for drinking purpose, and the water samples were subjected to systematic analysis with a view to understand the potability of drinking water sources. The values obtained for different parameters have been compared with the standard values given by ISI/ICMR/ WHO and the variations were notable for the parameters like electrical conductivity, total dissolved solids, total hardness and nitrates for few samples. Medical survey has been carried out to study the harmful effects on the society due to these four parameters at the areas - Tiruchanur, Renigunta and Karakambadi. Key words: Physico-chemical parameters; Total dissolved solids; Total hardness; Electrical conductivity. ______________________________________________________________________________ INTRODUCTION Water is one of the most essential components for the existence of life on earth. Although water pollution is an age- old problem, in this modern age, the problems like growing population, sewage disposal, industrial waste, radioactive waste, etc. have polluted our water resources so much that about 75 % rivers and streams, not only of India but also of all the countries, contain polluted waters [1].