Private Hospitals
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Indicators and Rationales for Health Care Reform in Malaysia
“1 Care for 1 Malaysia”?: Indicators and Rationales for Health Care Reform in Malaysia Dr. Por Heong Hong Contributor [email protected] 1. Introduction Since the attainment of independence in 1957, Malaysia’s health care service has been characterized by a mix of dominant public provision and significant presence of private services. Despite considerable changes over the past three decades, the dual system has made significant achievement with a total national expenditure less than the WHO recommended 5% GDP on health care each year. This is indicated in the country’s decreasing infant death rate, from 75.5 per 1,000 live births in 1957 to 6.8 per 1,000 live births in 2010, and improvement in life expectancy of male and female, from 55.8 years and 58.2 years in 1957 to 71.9 years and 77 years in 2010 respectively (see Table 1), REFSA which is above the world average of 68.5 years for males and 73.5 years for females over the period 2010–2013. Paper Focus Table 1: Infant death rate and life expectancy by sex in Malaysia, from 1957 to 2010. Infant death rate (per Life Expectancy (in years) Year 1,000 live births) Male Female 1957* 75.5 55.8 58.2 1980 19.7 66.7 71.6 2000 8.1 70.2 75.0 2010 6.8 71.9 77.0 1 Source: Vital Statistics Time Series: Peninsular Malaysia 1911-1985 by Department of Statistics, and Health Facts (various years) by Ministry of Health. Note: * Figure not inclusive of Sabah and Sarawak. -

Organizing Committee
ORGANIZING COMMITTEE 19th PSM CONGRESS ORGANIZING COMMITTEE Organizing Chairman : Dr Rosy Jawan Co-Organizing Chairman : Dr Soo Thian Lian Secretary : Dr See Kwee Ching Assistant Secretary : Dr Jumeah Shamsuddin Treasurer : Dr Neoh Siew Hong Fund Raising : Dr Irene Cheah Dr Bavanandan Naidu Sr Alice Ho Man Mooi Social Events : Dr Irene Cheah S/N Sangeeta a/p Rathanasamy Publications : Dr Alvin Chang Venue : Dr Irene Cheah Dr Rosy Jawan Sr Alice Ho Man Mooi Audio Visual : Dr Jumeah Samsudin 19th PSM CONGRESS SCIENTIFIC COMMITTEE Chairman : Dr Soo Thian Lian Co-Chairman : Dr Rosy Jawan Secretary : Dr See Kwee Ching Free Papers : Dr Bavanandan Naidu (O&G) Dr Chye Joon Kin (Neonatology) Sr Alice Ho Man Mooi (Nursing) Committee Members : Dr Jumeah Shamsuddin Dr Nazimah Idris Prof Dr Zaleha Mahdy Dr Irene Cheah Prof Dr Cheah Fook Choe Dr Neoh Siew Hong Sr Alice Ho Man Mooi Dr Alvin Chang (Representing PSS) 2 19th Annual PSM Perinatal Congress FACULTY OF SPEAKERS OVERSEAS FACULTY Andrew Ngu Terrence Thomas Consultant Obstetrician & Gynaecologist Consultant Chairman, Division of Obstetrics & Gynaecology Neurology Service Northern Hospital, Victoria Department of Paediatrics, Australia KK Women’s and Children’s Hospital Singapore Heather Jeffery Professor Victor Samuel Rajadurai International Maternal and Child Health Clinical Associate Professor School of Public Health, University of Sydney Head and Senior Consultant Clinical Academic Neonatologist Department of Neonatology Royal Prince Alfred Hospital, Sydney KK Women’s and Children’s Hospital -
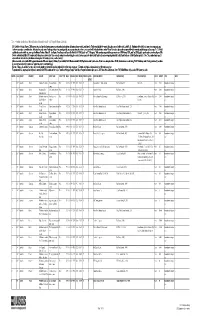
Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254)
Table1.—Attribute data for the map "Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254). [The United States Geological Survey (USGS) surveys international mineral industries to generate statistics on the global production, distribution, and resources of industrial minerals. This directory highlights the economically significant mineral facilities of Asia and the Pacific. Distribution of these facilities is shown on the accompanying map. Each record represents one commodity and one facility type for a single location. Facility types include mines, oil and gas fields, and processing plants such as refineries, smelters, and mills. Facility identification numbers (“Position”) are ordered alphabetically by country, followed by commodity, and then by capacity (descending). The “Year” field establishes the year for which the data were reported in Minerals Yearbook, Volume III – Area Reports: Mineral Industries of Asia and the Pacific. In the “DMS Latitiude” and “DMS Longitude” fields, coordinates are provided in degree-minute-second (DMS) format; “DD Latitude” and “DD Longitude” provide coordinates in decimal degrees (DD). Data were converted from DMS to DD. Coordinates reflect the most precise data available. Where necessary, coordinates are estimated using the nearest city or other administrative district.“Status” indicates the most recent operating status of the facility. Closed facilities are excluded from this report. In the “Notes” field, combined annual capacity represents the total of more facilities, plus additional -

Malaysia's Health 2004
Ministry of Health Malaysia MALAYSIA’S HEALTH 2004 Malaysia’s Health provides a glimpse of health developments in Malaysia, covering a wide array of topics on healthcare services, population health, health system management as well as research and development in the health sector in Malaysia. This technical report series was started in 1992 and provide the most comprehensive record of health developments in the country, including the private sector. Malaysia has a mix public-private healthcare system with the Ministry of Health as a major healthcare provider in the public sector. Being the lead agency for health, it provides leadership on matters relating to health, and sets the direction for health development in the country through standards and policy settings; promoting research and development in health; formulating health legislations and their enforcement to protect the health of its population; as well as providing high quality, accessible and affordable primary, secondary and tertiary care services to those who cannot afford private healthcare. Selected topics in this report provide an account of various initiatives undertaken by the Ministry of Health to improve health of its people, as well as healthcare services in its facilities and the coutry. These include quality improvements in healthcare provision and patient safety; affordable medicine; disease prevention and control; food quality control and safety; as well as health planning and management. The report on National Health Account gives an interesting insight into the pattern of health expenditures in the country while under the section on Research and Development, Malaysia’s role in establishing the Global Hub for Integrated Medicine and Herbal R&D is explained. -

What Lies Ahead for Malaysian Healthcare? Lee Poh Onn ISEAS – Yusof Ishak Institute E-Mail: [email protected]
No. 2015-4 What Lies Ahead for Malaysian Healthcare? Lee Poh Onn ISEAS – Yusof Ishak Institute E-mail: [email protected] ISEAS Economics Working Paper December 2015 Abstract Healthcare in Malaysia has been characterised by a strong public sector presence where government hospitals and clinics acted as a primary source of care. The healthcare system has also been lauded as a model for other developing countries to follow as it has succeeded in improving the health status of Malaysians over time. With the rising costs of healthcare over the last three decades, the government is now facing increasing pressures to restructure its healthcare system. Social healthcare insurance, corporatisation, and privatisation have been increasingly seen as possible measures to supplement the current healthcare system dominated by the public sector. In Malaysia, the involvement of government-linked companies in the private healthcare sector has, however, raised conflict-of-interest issues. Political economy factors will continue to play out; the private sector will continue to play an increasingly important role in the provision of healthcare while a long-awaited social healthcare insurance plan takes shape. Ultimately, clear rules of governance, regulations, and transparency have to be put in place to ensure that standards are maintained, conflict-of-interest issues are checked, and that healthcare continues to remain accessible. Keywords: Social Protection; Public Healthcare; Private Healthcare; Malaysia 30 Heng Mui Keng Terrace, Singapore 119614 6778 -

CHAPTER 1 INTRODUCTION 1.1 Healthcare in Malaysia Most Countries Undergoing Structural Change from Low to High Value Added Ac
CHAPTER 1 INTRODUCTION 1.1 Healthcare in Malaysia Most countries undergoing structural change from low to high value added activities of healthcare have been characterised by the private providers. The process of privatisation and contracting out services to the private businesses has led to an increasing shift in healthcare from being delivered as an essential public utility to a profit seeking target by private providers. Privatisation has also been used frequently by governments as a policy instrument to reduce the financial burden of the public sector. Malaysia is one of the countries that have experienced fundamental changes in the healthcare sector since independence in 1957. The colonial healthcare system in Malaya had originally been developed primarily for the purpose of serving the needs of the civil servants and other government employees and also the plantation sector (Harper, 1999), but expanded gradually to meet the needs of the general public. In addition, healthcare facilities were concentrated in urban areas with a focus on curative healthcare during the colonial period. After the Second World War, there were greater efforts to provide services to the rural areas both as part of overall development policies and as a strategic measure to counter Communist insurgency (Chee, 1990). The newly independent government of Malaya was committed to expanding state healthcare, especially services in rural areas has hitherto been neglected. At independence in 1957, the Federal Government assumed control of all government healthcare services, which had previously been, to a large extent, the responsibility of individual states of the Federation of Malaya (Barraclough, 1999). Government healthcare activities encompass curative, rehabilitative, promotive and regulatory concerns. -

Malaysia Health System Review Health Systems in Transition Vol
Health Systems in Transition Vol. 2 No. 1 2012 Vol. in Transition Health Systems Health Systems in Transition Vol. 3 No.1 2013 Malaysia Health System Review The Asia Pacific Observatory on Health Review Malaysia Health System Systems and Policies is a collaborative partnership which supports and promotes evidence-based health policy making in the Asia Pacific Region. Based in WHO’s Regional Office for the Western Pacific it brings together governments, international agencies, foundations, civil society and the research community with the aim of linking systematic and scientific analysis of health systems in the Asia Pacific Region with the decision- makers who shape policy and practice. Asia Pacific Observatory on Health Systems and Policies Health Systems in Transition Vol. 3 No. 1 2013 Malaysia Health System Review Written by: Safurah Jaafar, Ministry of Health, Malaysia Kamaliah Mohd Noh, Ministry of Health, Malaysia Khairiyah Abdul Muttalib, Ministry of Health, Malaysia Nour Hanah Othman, Ministry of Health, Malaysia Judith Healy, Australian National University, Australia Other authors: Kalsom Maskon, Ministry of Health, Malaysia Abdul Rahim Abdullah, Ministry of Health, Malaysia Jameela Zainuddin, Ministry of Health, Malaysia Azman Abu Bakar, Ministry of Health, Malaysia Sameerah Shaikh Abd Rahman, Ministry of Health, Malaysia Fatanah Ismail, Ministry of Health, Malaysia Chew Yoke Yuen, Ministry of Health, Malaysia Nooraini Baba, Ministry of Health, Malaysia Zakiah Mohd Said, Ministry of Health, Malaysia Edited by: Judith Healy, Australian National University, Australia WHO Library Cataloguing in Publication Data Malaysia health system review. (Health Systems in Transition, Vol. 2 No. 1 2012) 1. Delivery of healthcare. 2. Health care economics and organization. -

Process Flow Improvement Using Value Stream Mapping to Reduce Waste and Lead Time in Malaysia Healthcare Siti Haizatul Aishah Bt
PROCESS FLOW IMPROVEMENT USING VALUE STREAM MAPPING TO REDUCE WASTE AND LEAD TIME IN MALAYSIA HEALTHCARE SITI HAIZATUL AISHAH BT HARON UNIVERSITI TUN HUSSEIN ONN MALAYSIA PROCESS FLOW IMPROVEMENT USING VALUE STREAM MAPPING TO REDUCE WASTE AND LEAD TIME IN MALAYSIA HEALTHCARE SITI HAIZATUL AISHAH BT HARON A thesis submitted in fulfilment of the requirement for the award of the Degree of Master of Science in Technology Management Faculty of Technology Management and Business Universiti Tun Hussein Onn Malaysia FEBRUARY 2017 iii DEDICATION I dedicate this thesis to Almighty ALLAH S.W.T, My father (Haron Bin Mohd Saad), my mother (Zainah Binti Buang) and siblings, For your love, care and encouragement. My supervisor and co-supervisor, For your help, encouragement and guidance to ensure the success of this thesis. Friends, For your help, encouragement, helped me through, make me feel like I am not alone, may Allah ease their journey and never give up. And everyone who involves directly and indirectly in the process of completing this thesis. Thank you. iv ACKNOWLEDGEMENTS All praise to God, the Greatest that gives perfection and facility in applying all tasks and responsibilities. I would like to acknowledge the generous contribution of individuals and organisations to this research, without them this research would not have been successfully completed. First and foremost, I would like to express my gratitude to my supervisor, Puan Rohaizan Binti Ramlan, my co-supervisor, Dr Kamilah Binti Ahmad, and lecturer, Dr Ahmad Aizat Bin Ahmad for providing me with invaluable guidance, inspiration, encouragement for my research and for being a mentor and supporter with their constant inspiration. -

Electricity & Gas Supply Infrastucture Malaysia
ELECTRICITY & GAS SUPPLY INFRASTRUCTURE MALAYSIA LSS2 Projects Status (Peninsular Malaysia) (Commercial Operation Date end 2019 - TBC) LSS2 Projects Status (Peninsular Malaysia) (Commercial Operation Date 2020 - TBC) PENINSULAR MALAYSIA No. Solar Power Producer (SPP) Plant Capacity (MW) Plant Location No. Solar Power Producer (SPP) Plant Capacity (MW) Plant Location MAP 2 SABAH & SARAWAK JDA A-18 1. Solution Solar 1 Sdn Bhd 4.00 Port Klang, Selangor 14. Scope Marine Sdn Bhd 5.00 Setiu, Terengganu SESB SJ- Melawa (DG 324MW, GT 20MW) Ranhill Powertron II (GT&ST) 214.8MW LSS1 Projects Status (Sabah) 2. Jentayu Solar Sdn Bhd 5.99 Pokok Sena, Kedah 15. Hong Seng Assembly Sdn Bhd 1.00 Seberang Perai Utara, Pulau Pinang No. Solar Power Producer (SPP) Plant Capacity (MW) Plant Location Karambunai Gayang 3. Solution Solar 2 Sdn Bhd 3.00 Port Klang, Selangor 16. Coral Power Sdn Bhd 9.99 Manjung, Perak Kayumadang Ranhill Powertron I (Teluk Salut) CCGT 208.64MW 1. Sabah Energy Corporation Sdn Bhd 5.00 Wilayah Persekutuan Labuan JDA B-17 4. Fairview Equity Project (Mersing) Sdn Bhd 5.00 Mersing, Johor 17. I2 Solarpark One Sdn Bhd 6.80 Alor Gajah, Melaka Unggun 2. Nusantara Suriamas Sdn Bhd 5.90 Kota Marudu, Sabah Sepanggar Bay (GT&ST) 113.8MW 5. Maju Solar (Gurun) Sdn Bhd 9.90 Kuala Muda, Kedah 18. Viva Solar Sdn Bhd 30.00 Sik, Kedah 3. Beau Energy East Sdn Bhd 6.00 Beaufort, Sabah 6. Asia Meranti Solar (Kamunting) Sdn Bhd 9.90 Kamunting, Perak 19. Cypark Estuary Solar Sdn Bhd 30.00 Empangan Terip, Negeri Sembilan UMS2 7. -

Krinstitute.Org
SOCIAL INEQUALITIES AND HEALTH IN MALAYSIA THE STATE OF HOUSEHOLDS 2020 PART III SOCIAL INEQUALITIES AND HEALTH IN MALAYSIA THE STATE OF HOUSEHOLDS 2020 PART III ©2020 Khazanah Research Institute December 2020 Social Inequalities and Health in Malaysia: The State of Households 2020 Part III. – Kuala Lumpur, Malaysia: Khazanah Research Institute This work is available under the Creative Commons Attribution 3.0 Unported license (CC BY3.0) http://creativecommons.org/licenses/by/3.0/. Under the Creative Commons Attribution license, you are free to copy, distribute, transmit, and adapt this work, including for commercial purposes, under the following attributions: Attribution – Please cite the work as follows: Khazanah Research Institute. 2020. Social Inequalities and Health in Malaysia: The State of Households 2020 Part III. Kuala Lumpur: Khazanah Research Institute. License: Creative Commons Attribution CC BY 3.0. Translations – If you create a translation of this work, please add the following disclaimer along with the attribution: This translation was not created by Khazanah Research Institute and should not be considered an official Khazanah Research Institute translation. Khazanah Research Institute shall not be liable for any content or error in this translation. Published December 2020. Published by Khazanah Research Institute at Level 25, Mercu UEM, Jalan Stesen Sentral 5, Kuala Lumpur Sentral 50470 Kuala Lumpur, Malaysia. Fax: +603 2265 0088; email: [email protected] All queries on rights and licenses should be addressed to the Chairman’s Office, Khazanah Research Institute at the address stated above. Information on Khazanah Research Institute publications and digital products can be found at www.KRInstitute.org Cover artwork by Wan Amirah Wan Usamah, based on photo by Lightscape on Unsplash. -

Contemporary Healthcare Experience in Malaysian Hospitals
Contemporary Healthcare Experience in Malaysian Hospitals Hasliza Hassan Multimedia University, Cyberjaya, Selangor, Malaysia Muhammad Sabbir Rahman International Islamic University Malaysia, Kuala Lumpur, Malaysia Abu Bakar Sade UCSI University, Kuala Lumpur, Malaysia The main dissatisfaction of patients in hospital is the long waiting time, which could lead to stress. This research makes suggestions concerning how to reduce the impact of the negative perception about the lengthy waiting time by having a contemporary healthcare experience through sub-retailers and nursery services within the hospital. It is highly expected that the enhancement of the basic healthcare concept through sub-retailers within the hospital will create a more relaxed feeling while waiting for medical treatment. In addition, it is suggested that nursery services would also provide more convenience for the patients. INTRODUCTION Healthcare possesses a key momentum to push the development of a nation, as healthy nations will have a more progressive impact on economic development, which could be shared by the society. The largest healthcare service in Malaysia is provided by the Ministry of Health (Hazilah, 2009; Manaf, 2005). The other two ministries that offer a healthcare service are the Ministry of Education and the Ministry of Defense (Manaf, 2005). Overall, 53% of the total government allocation for healthcare is being used by the Ministry of Health (Yon, 2002). The three categories of public hospitals that are provided by the Ministry of Health are at the national, state and district levels (Manaf, 2005). Kuala Lumpur Hospital is the largest public hospital in Malaysia (Hazilah, 2009). Approximately 92% of the population in urban areas stays within 3 km of a healthcare facility while only 67% of the population in rural areas enjoy a similar benefit (Suleiman and Jegathesan, 2000). -

Curriculum Vitae
CURRICULUM VITAE Dr Vijaya B Ramasamy MBChB (Dundee), MRCP (UK), SCE Nephrology (UK), CCT (UK), FRCP (London) I am a Consultant Nephrologist and Physician in Lam Wah Ee Hospital (LWEH), Penang since my relocation back to Malaysia in August 2018. I am also the current lead for medical mortality and morbidity at LWEH. Prior to the current appointment, I was a Consultant Nephrologist and Physician at the Wrexham Maelor Hospital (WMH), UK since 2015. Having qualified from University of Dundee with Bachelors of Medicine and Surgery (MBChB) in 2006, I completed my internship in Dundee, UK before taking up medical resident post at Cardiff, UK in 2008. I completed my MRCP and subsequently undertook specialist training in Nephrology and Internal Medicine at University Hospital of Wales, Cardiff and WMH between 2010 and 2015. I passed the Specialist Certificate Examination in Renal Medicine in 2013 (SCE Nephrology) and obtained Certificate of Completion of Training (CCT) in Nephrology and Internal Medicine in 2015. I was entered onto the GMC UK Specialist Register for Nephrology and General Internal Medicine in 2015 and still remain in the register as a specialist in the UK. I am also registered with the Malaysian National Specialist Register (NSR) for both Nephrology & Internal Medicine. My subspecialty interest includes hypertension, acute kidney injury (AKI), interventional nephrology / vascular access and transplantation, with active involvement in quality improvement projects and research in these fields. 2018 Fellowship of the Royal College of Physicians of London (FRCP) 2015 Certificate of Completion of Training (CCT) Renal Medicine – General Medical Council UK 2015 Certificate of Completion of Training (CCT) General (Internal) Medicine – General Medical Council UK 2013 Specialist Certificate Examination (SCE) in Nephrology 2011 Membership of the Royal Colleges of Physicians of the United Kingdom (MRCP) 2006 Bachelor of Medicine and Bachelor of Surgery (MBChB) - University of Dundee, UK .