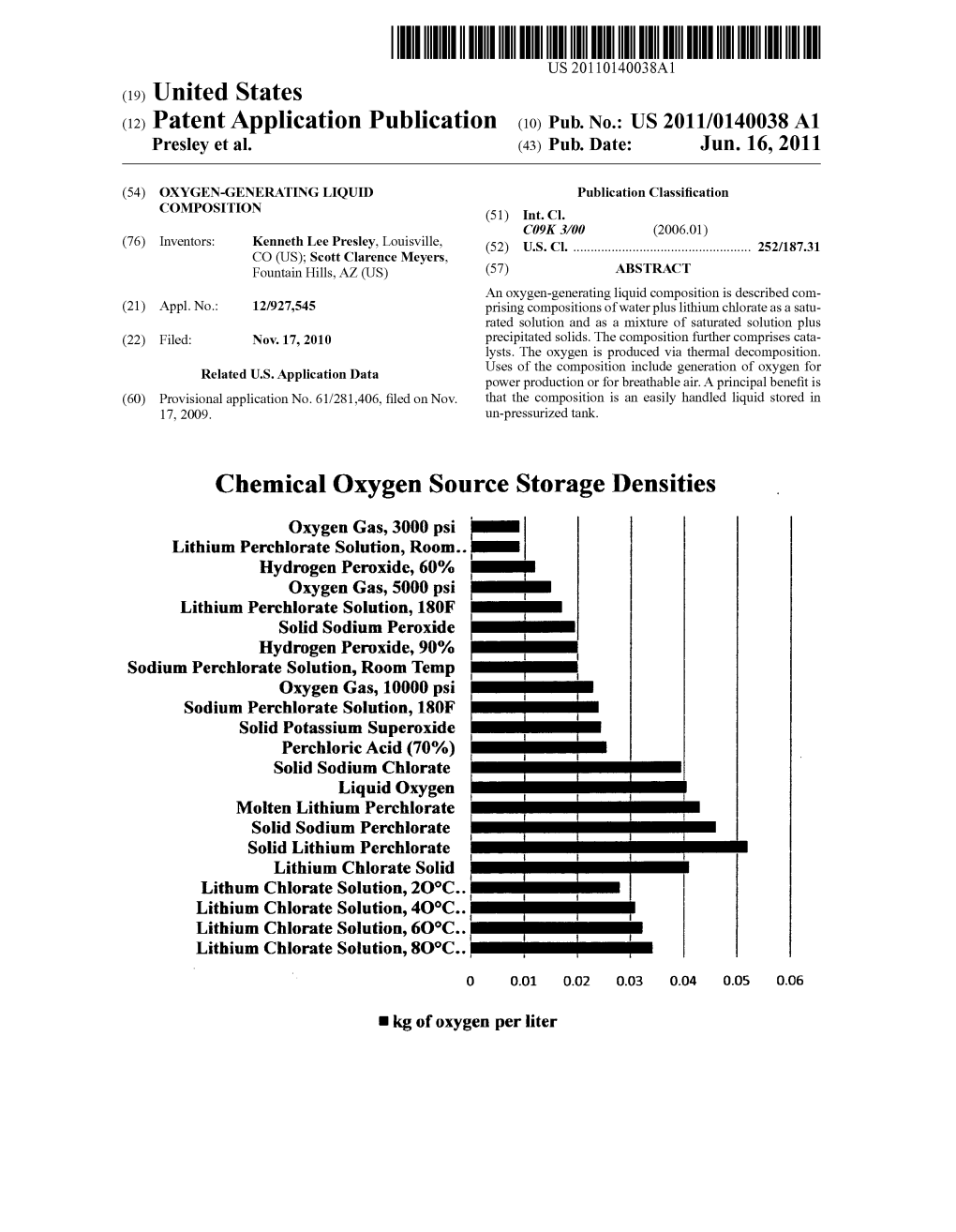
Chemical Oxygen Source Storage Densities Oxygen Gas, 3000 Psi Lithium Perchlorate Solution, Room
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Article the Oxygen Generation Performance of Hollow-Structured Oxygen Candle for Refuge Space
Hindawi Journal of Chemistry Volume 2018, Article ID 7469783, 9 pages https://doi.org/10.1155/2018/7469783 Research Article The Oxygen Generation Performance of Hollow-Structured Oxygen Candle for Refuge Space Weixiang Wang,1,2 Longzhe Jin,1,2 Na Gao ,1,2 Jianlin Wang,1 and Mingyang Liu1 1School of Civil and Resource Engineering, University of Science and Technology Beijing, Beijing 100083, China 2Mine Emergency Technology Research Center, Beijing 100083, China Correspondence should be addressed to Na Gao; [email protected] Received 26 May 2018; Revised 27 August 2018; Accepted 22 October 2018; Published 2 December 2018 Academic Editor: Albert Demonceau Copyright © 2018 Weixiang Wang et al. ,is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To improve oxygen generation performance, we dissected and analyzed the incompletely reacted oxygen candles and thus proposed the concept of the hollow-structured oxygen candle. We calculated the surface area ratio and designed the mold for hollow-structured oxygen candles at a radius of 0, 5, 9, 12, 15.5, and 20 mm. ,e structural stability of the oxygen candles was tested by the loading experiment. ,e oxygen generation rate (OGR) and other properties were explored by combustion ex- periments. ,e composition of the oxygen candles and the residual solids after combustion were observed with scanning electron microscope (SEM). ,e results show that, with the increase of the hollow-structure radius (r), the stability of the hollow-structured oxygen candles gradually weakens, and the oxygen candles cannot be made when r is 20 mm. -

Analysis of the Thermal Behaviour of CL-20, Potassium Perchlorate, Lithium Perchlorate and Their Admixtures by DSC and TG
Central European Journal of Energetic Materials ISSN 1733-7178; e-ISSN 2353-1843 Copyright © 2018 Institute of Industrial Organic Chemistry, Poland Cent. Eur. J. Energ. Mater. 2018, 15(1): 115-130; DOI: 10.22211/cejem/78089 Analysis of the Thermal Behaviour of CL-20, Potassium Perchlorate, Lithium Perchlorate and Their Admixtures by DSC and TG Jing-yuan Zhang, Xue-yong Guo,* Qing-jie Jiao, Hong-lei Zhang, Hang Li State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China * E-mail: [email protected] Abstract: The thermal decomposition characteristics of CL-20, potassium perchlorate (KP), lithium perchlorate (LP), a CL-20/KP mixture, and a CL-20/LP mixture were studied using thermogravimetry-differential scanning calorimetry (TG-DSC). The DSC curves for KP exhibited three endothermic peaks and one exothermic peak. The first two endothermic peaks correspond to the rhombic- cubic transition and the fusion of KP, respectively, the third indicates the fusion of KCl, while the exothermic peak is attributed to the decomposition of KP. The DSC curves obtained from LP showed four endothermic peaks and one exothermic peak. The first two endothermic peaks indicate the loss of adsorbed water and water of crystallization, while the third and fourth are associated with the fusion of LP and LiCl, respectively; the exothermic peak is due to the decomposition of LP. The presence of KP had little effect on the thermal decomposition of CL-20 while the addition of LP increased the temperature at which CL-20 exhibits an exothermic peak. In addition, the thermal decomposition of LP appeared to be catalyzed by the presence of CL-20. -

Breakthrough Chemistry Simulations for Lithium Processing Contents
think simulation | getting the chemistry right Breakthrough chemistry simulations for lithium processing Using simulation to maximize your investment return in lithium extraction and processing Contents Introduction ............................................................................................................................................ 2 Process simulation deficiencies ................................................................................................................ 2 OLI Systems electrolyte thermodynamics .................................................................................................2 OLI Systems lithium initiative .................................................................................................................... 2 Lithium phase 1 and potash chemistry is complete ................................................................................... 2 Fundamental sulfate – chloride systems .............................................................................................. 3 Fundamental hydroxide and carbonate systems .................................................................................. 3 Lithium in acid environments for processing and recycling ................................................................... 3 Lithium borate systems for Li production ............................................................................................. 4 Systems related to Li hydrometallurgical processing, purifications and recycling .................................. -

Polyorganosiloxanes: Molecular Nanoparticles, Nanocomposites and Interfaces
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses November 2017 POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES Daniel H. Flagg University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Materials Chemistry Commons, Polymer and Organic Materials Commons, and the Polymer Chemistry Commons Recommended Citation Flagg, Daniel H., "POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES" (2017). Doctoral Dissertations. 1080. https://doi.org/10.7275/10575940.0 https://scholarworks.umass.edu/dissertations_2/1080 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES A Dissertation Presented by Daniel H. Flagg Submitted to the Graduate School of the University of Massachusetts in partial fulfillment of the degree requirements for the degree of DOCTOR OF PHILOSOPHY September 2017 Polymer Science and Engineering © Copyright by Daniel H. Flagg 2017 All Rights Reserved POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES A Dissertation Presented by Daniel H. Flagg Approved as to style and content by: Thomas J. McCarthy, Chair E. Bryan Coughlin, Member John Klier, Member E. Bryan Coughlin, Head, PS&E To John Null ACKNOWLEDGEMENTS There are countless individuals that I need to thank and acknowledge for getting me to where I am today. I could not have done it alone and would be a much different person if it were not for the support of my advisors, friends and family. -

Quantification of Lithium Via Redox Titration and Ph Titration – a Method Comparison
Quantification of Lithium via Redox Titration and pH Titration – A Method Comparison by Joseph J. Hebert A THESIS submitted to Oregon State University Honors College in partial fulfillment of the requirements for the degree of Honors Baccalaureate of Science in Chemical Engineering and Chemistry (Honors Associate) Presented May 26, 2020 Commencement June 2020 AN ABSTRACT OF THE THESIS OF Joseph J. Hebert for the degree of Honors Baccalaureate of Science in Chemical Engineering and Chemistry presented on May 26, 2020. Title: Quantification of Lithium via Redox Titration and pH Titration - A Method Comparison. Abstract approved:_____________________________________________________ Michael Lerner Lithium serves an unparalleled role for high energy-density storage applications and is vital for the continued advancement of the world economy. However, global supply is heavily reliant on lithium deposits situated in select locations, creating unpredictability in the price and concerns for the sustained production of the resource. Additionally, future demands for applications in the small electronics, automotive, and renewable energy industries threaten to place further strain on the lithium supply. Thus, the implementation of lithium battery recycling methods is critical meet this expected surge in demand for lithium-based battery technologies. Several economic obstacles and safety considerations have halted the advancement of these necessary recycling techniques. A prominent barrier to recycling efforts revolves around the reactivity of active lithium compounds that remain in used lithium batteries. As a result, significant safety precautions must be taken when handling and transporting lithium-based batteries, adding to the costs associated with recycling methods. Current research has been dedicated to developing a passivation method for the remaining active lithium in used cells, seeking to lower the classification, and subsequently the costs, associated with these materials. -

Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: a Review
resources Review Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review Laurence Kavanagh * , Jerome Keohane, Guiomar Garcia Cabellos, Andrew Lloyd and John Cleary EnviroCORE, Department of Science and Health, Institute of Technology Carlow, Kilkenny, Road, Co., R93-V960 Carlow, Ireland; [email protected] (J.K.); [email protected] (G.G.C.); [email protected] (A.L.); [email protected] (J.C.) * Correspondence: [email protected] Received: 28 July 2018; Accepted: 11 September 2018; Published: 17 September 2018 Abstract: Lithium is a key component in green energy storage technologies and is rapidly becoming a metal of crucial importance to the European Union. The different industrial uses of lithium are discussed in this review along with a compilation of the locations of the main geological sources of lithium. An emphasis is placed on lithium’s use in lithium ion batteries and their use in the electric vehicle industry. The electric vehicle market is driving new demand for lithium resources. The expected scale-up in this sector will put pressure on current lithium supplies. The European Union has a burgeoning demand for lithium and is the second largest consumer of lithium resources. Currently, only 1–2% of worldwide lithium is produced in the European Union (Portugal). There are several lithium mineralisations scattered across Europe, the majority of which are currently undergoing mining feasibility studies. The increasing cost of lithium is driving a new global mining boom and should see many of Europe’s mineralisation’s becoming economic. The information given in this paper is a source of contextual information that can be used to support the European Union’s drive towards a low carbon economy and to develop the field of research. -

Oxygen Carriers
Oxygen Carriers Prof. Ramesh Chandra Department of Chemistry University of Delhi The main function of red blood cell is - Transfer of O2 from lungs to tissue. Transfer of CO2 from tissue to lungs. To accomplish this function red cells has Oxygen carriers. All aerobic forms of life depends on Oxygen Carriers. The transport and storage of Oxygen is extremely important physiological function and this is done by oxygen carriers. Various types of oxygen carriers occurring in living systems are - Oxygen Found in Metal Present Function carriers Hemoglobin All Mammals Fe(II) Carrier (Hb) Hemerythrin Various marine Fe(III) Carrier (Hr) invertebrates Myoglobin All Mammals Fe(II) Storage (Mb) Hemocyanin Arthropods & Cu(II) Carrier (Hc) Molluscs Dioxygen as Ligand Under appropriate circumstances, Dioxygen molecule become a ligand and this process is called OXYGENATION in which oxygen molecule retains its identity. 1 In ground state of oxygen molecule, it has two unpaired electrons in *(2py) and 1 *(2px) antibonding molecular orbitals. The degeneracy of -molecular orbital leads to Biradical Character of oxygen molecule. The binding of dioxygen by a transition metal involves electron transfer reaction from metal to dioxygen forming a coordinated superoxide ion. The coordinated superoxide ion binds to the metal in an angular as opposed to a perpendicular fashion and usually has a partial negative charge concentrated on the terminal oxygen atom. This superoxide ligand may be stabilized by an electrophile in the distal cavity, for example, the formation of a hydrogen bond. What are the Oxygen Carriers?? Oxygen carriers are compounds which can take up and release the oxygen reversibly. -

Synthetic Oxygen Carriers Related to Biological Systems
Synthetic Oxygen Carriers Related to Biological Systems ROBERT D. JONES, DAVID A. SUMMERVILLE, and FRED BASOLO" Department of Chemistry, North western University, E vanston, lllinois 6020 1 Received September 29, 1978 Confenfs early part of 1978, no attempt has been made to totally cover all aspects of the problem. Rather we have tried to approach the I. Introduction 139 subject in such a way as to complement the other reviews al- II. Nomenclature 139 ready available.2-18Specifically we have largely restricted our 111. Nature of 02 139 attention to the synthetic approaches that have been made to IV. Nature of Bound Dioxygen 140 observe metastable metal-dioxygen complexes of biological V. Natural Oxygen Carriers 143 importance. A. Heme-Containing Proteins, Hb and Mb 143 B. Hemerythrin 146 /I. Nomenclafure C. Hemocyanin 146 VI. Synthetic 02 Carriers 147 The terminology used in this review, referring to oxygen in its A. Porphyrins 147 various forms, is similar to that used by Vaskai5 in his recent B. Schiff Bases 148 review. The term molecular oxygen as used here refers only to VII. Cobalt-Dioxygen Carriers 148 the free uncombined O2 molecule. Unless otherwise specified, A. Early Work 148 it will be used to mean the ground (32g-)state of the molecule. 6. Recent Studies on Cobalt-Dioxygen Complexes The term dioxygen has been used as a generic designation for in Nonaqueous Solutions 149 the O2 moiety in any of its several forms, and can refer to 02 in C. Cobalt-Dioxygen Complexes in either a free or combined state. The only criterion for use of this Aqueous Solution 160 term is the presence of a covalent bond between the oxygen D. -

Procedures for Trace Analysis of Dissolved Inorganic and Organic Constituents in Water Digital Object Identifier
University of Kentucky UKnowledge KWRRI Research Reports Kentucky Water Resources Research Institute 1971 Procedures for Trace Analysis of Dissolved Inorganic and Organic Constituents in Water Digital Object Identifier: https://doi.org/10.13023/kwrri.rr.42 Gary D. Christian University of Kentucky Charles E. Matkovich University of Kentucky W. Lynn Schertz University of Kentucky Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/kwrri_reports Part of the Inorganic Chemicals Commons, Organic Chemicals Commons, and the Water Resource Management Commons Repository Citation Christian, Gary D.; Matkovich, Charles E.; and Schertz, W. Lynn, "Procedures for Trace Analysis of Dissolved Inorganic and Organic Constituents in Water" (1971). KWRRI Research Reports. 153. https://uknowledge.uky.edu/kwrri_reports/153 This Report is brought to you for free and open access by the Kentucky Water Resources Research Institute at UKnowledge. It has been accepted for inclusion in KWRRI Research Reports by an authorized administrator of UKnowledge. For more information, please contact [email protected]. PROCEDURES FOR TRACE ANALYSIS OF DISSOLVED INORGANIC AND ORGANIC CONSTITUENTS IN WATER Gary D. Christian Principal Investigator Charles E. Matkovich W. Lynn Schertz Research Assistants University of Kentucky Water Resources Institute Lexington, Kentucky WASHINGTON WA-TSII RESEARCH CENTl!li uaiiwtv Project Number A-013-KY (Completion Report) Agreement Nos. 14-01-0001-1636 14-31-0001-3017 July 1968 - June 1971 The work upon which this report is based was supported by funds provided by the United States Department of the Interior, Office of Water Resources Research, as authorized under the Water Resources Act • of 1964. -

Safety Data Sheet Acc
Page 1 of 13 Safety Data Sheet acc. to OSHA HCS (29 CFR 1910.1200) Printing date 01/22/2015 Reviewed on 01/22/2015 1 Identification · Product identifier · Trade name: Li-MnO2 Button Cell · Article number: CR2032 · Application of the substance / the mixture Lithium-based battery product. · Details of the supplier of the Safety Data Sheet · Manufacturer/Supplier: Jintan Chaochuang Battery Company Limited Xiyang Industrial Estate, Maolu Town Jintan City, Jiangsu Province, China Phone: +86-519-82483588 Fax: +86-755-29369623 · Emergency telephone number: +86-519-82483588 2 Hazard(s) identification · Classification of the substance or mixture GHS02 Flame Water-react. 3 H261 In contact with water releases flammable gas. · Additional information: There are no other hazards not otherwise classified that have been identified. 0 percent of the mixture consists of ingredient(s) of unknown toxicity. · Label elements · GHS label elements The product is classified and labeled according to the Globally Harmonized System (GHS). · Hazard pictograms GHS02 · Signal word Warning · Hazard-determining components of labeling: lithium · Hazard statements H261 In contact with water releases flammable gas. · Precautionary statements P280 Wear protective gloves and eye protection. P370+P378 In case of fire: Use for extinction: Fire-extinguishing powder. P402+P404 Store in a dry place. Store in a closed container. P501 Dispose of contents/container in accordance with local/regional/national/international regulations. · Additional information: Information references exposures to battery contents, and not exposures to whole units. Exposures to whole units are unlikely to produce health hazards. (Contd. on page 2) 40.0.6 Page 2 of 13 Safety Data Sheet acc. -

Lithium Perchlorate
Lithium perchlorate sc-215260 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Lithium perchlorate STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY0 HEALTH2 HAZARD INSTABILITY2 OX SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS Cl-Li-O4, LiClO4, "perchloric acid, lithium salt" Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability 0 Toxicity 2 Min/Nil=0 Body Contact 2 Low=1 Reactivity 2 Moderate=2 High=3 Chronic 2 Extreme=4 CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Contact with combustible material may cause fire. Irritating to eyes, respiratory system and skin. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ■ Accidental ingestion of the material may be damaging to the health of the individual. ■ Lithium, in large doses, can cause dizziness and weakness. If a low salt diet is in place, kidney damage can result. ■ Symptoms of exposure to perchlorates include shortness of breath, difficulty breathing and a bluish discolouration of the skin. The effects may be delayed for several hours following exposure. ■ Nausea and vomiting are almost always apparent after chlorate poisonings usually with upper stomach pain. Diarrhoea may also occur. EYE ■ This material can cause eye irritation and damage in some persons. SKIN ■ This material can cause inflammation of the skin oncontact in some persons. -

Safety Standard for Oxygen and Oxygen Systems
NSS 1740.15 JANUARY 1996 National Aeronautics and Space A_tration SAFETY STANDARD FOR OXYGEN AND OXYGEN SYSTEMS Guidelines for Oxygen System Design, Materials Selection, Operations, Storage, and Transportation Office of Safety and Mission Assurance Washington, DC 20546 Safety Standard for Oxygen and Oxygen Systems Guidelines for Oxygen System Design, Materials Selection, Operations, Storage, and Transportation PREFACE This safety standard establishes a uniform Agency process for oxygen system design, materials selection, operation, storage, and transportation. This standard contains minimum guidelines applicable to NASA Headquarters and all NASA Field Installations. Installations are encouraged to assess their individual programs and develop additional requirements as needed. "Shalls" and "wills" denote requirements that are mandated in other existing documents referenced at the end of each chapter and in widespread use in the aerospace industry. This standard is issued in loose-leaf form and will be revised by change pages. Comments and questions concerning the contents of this publication should be referred to the National Aeronautics and Administration Headquarters, Director, Safety and Risk Management Division, Office of the Associate for Safety and Mission Assurance, ashington, DC 20546. EFFECTIVE DATE: JAN 3 0 1996 Safety and Mission Assurance ACKNOWLEDGEMENTS The NASA Oxygen Safety Handbook was originally prepared under NASA contract NAS3- 23558 by Paul M. Ordin, Consulting Engineer. The support of the NASA Hydrogen-Oxygen Safety Standards Review Committee in providing technical monitoring of the standard is gratefully acknowledged. The committee included the following members: William J. Brown (Chairman) NASA Lewis Research Center Cleveland, Ohio Frank J. Benz NASA Johnson Space Center White Sands Test Facility Las Cruces, New Mexico Mike Pedley NASA Johnson Space Center Houston, Texas Dennis Griffin NASA Marshall Space Flight Center Alabama Coleman J.