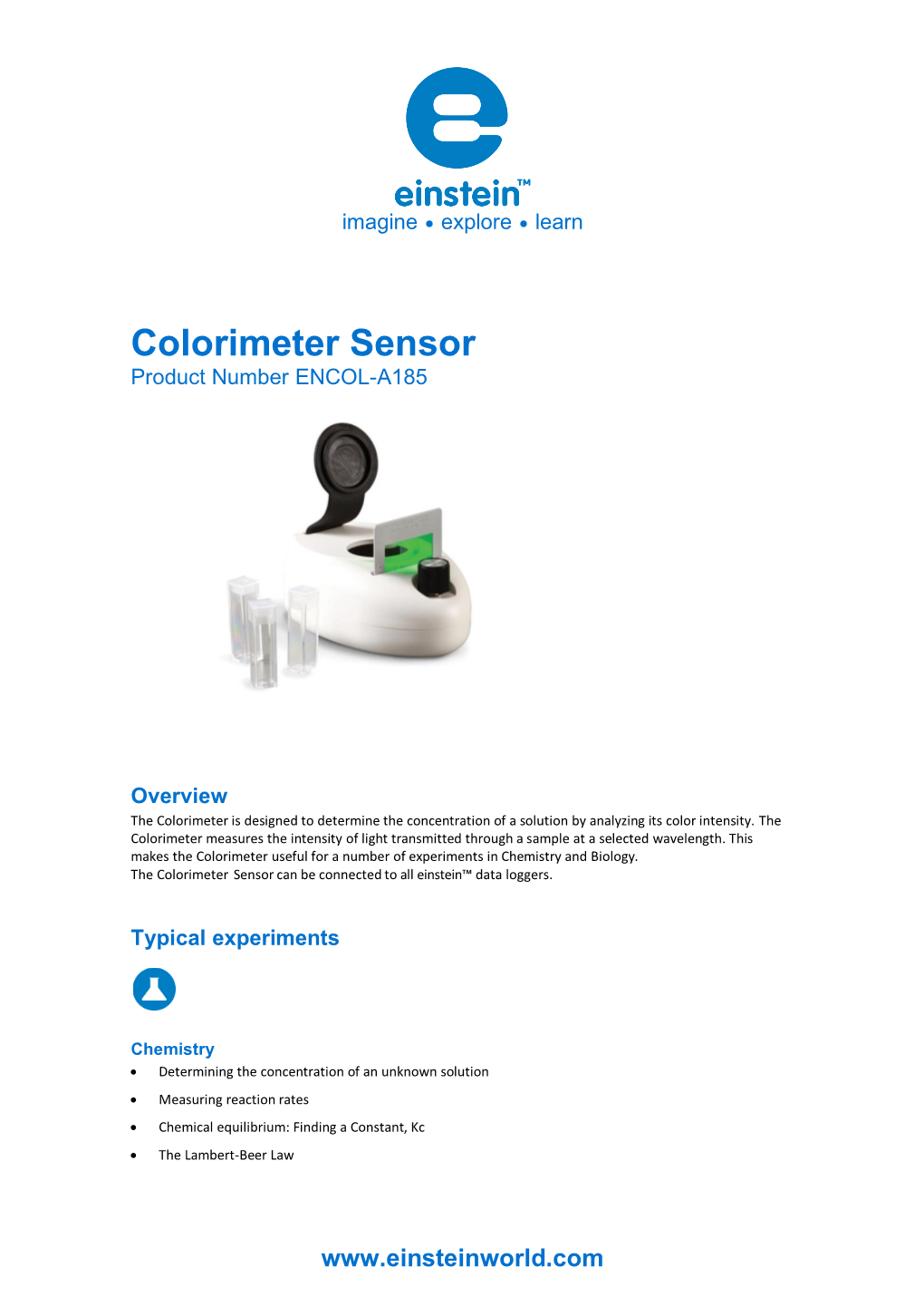
Colorimeter Sensor Product Number ENCOL-A185
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nanodrop 2000/2000C Spectrophotometers
NanoDrop 2000/2000c Spectrophotometer V1.0 User Manual The information in this publication is provided for reference only. All information contained in this publication is believed to be correct and complete. Thermo Fisher Scientific shall not be liable for errors contained herein nor for incidental or consequential damages in connection with the furnishing, performance or use of this material. All product specifications, as well as the information contained in this publication, are subject to change without notice. This publication may contain or reference information and products protected by copyrights or patents and does not convey any license under our patent rights, nor the rights of others. We do not assume any liability arising out of any infringements of patents or other rights of third parties. We make no warranty of any kind with regard to this material, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Customers are ultimately responsible for validation of their systems. © 2009 Thermo Fisher Scientific Inc. All rights reserved. No part of this publication may be stored in a retrieval system, transmitted, or reproduced in any way, including but not limited to photocopy, photograph, magnetic or other record, without our prior written permission. For Technical Support, please contact: Thermo Fisher Scientific 3411 Silverside Road Bancroft Building, Suite 100 Wilmington, DE 19810 U.S.A. Telephone: 302-479-7707 Fax: 302-792-7155 E-mail: [email protected] www.nanodrop.com For International Support, please contact your local distributor. Microsoft, Windows, Windows NT and Excel are either trademarks or registered trademarks of Microsoft Corporation in the United States and/or other countries. -

WPA CO 7500 Colorimeter User Manual
WPA CO 7500 Colorimeter User Manual Biochrom Ltd Certificate No. 890333 Declaration of Conformity This is to certify that the WPA CO 7500 Colorimeter Part number 80-3000-43 (mains only) 80-3000-44 (mains / battery) manufactured by Biochrom Ltd. conform to the requirements of the following Directives-: 73/23/EEC & 89/336/EEC Standards to which conformity is declared EN 61 010-1: 2001 Safety requirements for electrical equipment for measurement, control and laboratory use. EN 61326: 1998 Electrical equipment for measurement, control and laboratory use – EMC requirements Signed: Dated: 24th March 2003 David Parr Managing Director Biochrom Ltd Postal address Telephone Telefax Biochrom Ltd +44 1223 423723 +44 1223 420164 22 Cambridge Science Park Milton Road e mail: [email protected] website: http://www.biochrom.co.uk Cambridge CB4 0FJ England Registered in England No: 3526954 Registered Office: 22 Cambridge Science Park, Milton Road, Cambridge CB4 0FJ, England. CONTENTS Unpacking, Positioning and Installation 1 OPERATION 2 Introduction 2 Using the Instrument 3 Making an absorbance or %T measurement 4 Making a kinetics measurement 4 TROUBLE SHOOTING NOTES 5 ACCESSORIES, CONSUMABLES AND SPARES 6 OUTPUT OF RESULTS 6 Use with serial printer 6 Use with PC 6 Use with chart recorder 6 MAINTENANCE 7 General maintenance 7 Changing a filter 7 Replacing the light bulb 8 SPECIFICATION AND WARRANTY 9 Unpacking, Positioning and Installation • Ensure your proposed installation site conforms to the environmental conditions for safe operation: Indoor use only Temperature 5°C to 35°C Maximum relative humidity of 80 % up to 31°C decreasing linearly to 50 % at 40°C If this equipment is used in a manner not specified or in environmental conditions not appropriate for safe operation, the protection provided by the equipment may be impaired and instrument warranty withdrawn. -

Bluvision A4 New 2018.Indd
The BluVision™ Discrete analyzer your partner in chemistry automation Introduction Skalar launches its new automation Parameters: technology, the BluVision™ • Alkalinity • Free Aluminum discrete analyzer, for the analysis of • Ammonia colorimetric parameters. • Calcium • Chloride With the BluVision™ discrete analyzer we • Chromium VI complement our range of products for the • Color automation of colorimetric analysis, which already • Free Cyanide consists of the San++ segmented fl ow analyzer and the SP2000 test kit robotic analyzer. • Total Hardness • Free Iron The Discrete analyzer is ideal for environmental • Magnesium and industrial laboratories analyzing a wide • Nitrate+Nitrite variety of sample types and matrices. This system • Nitrite integrates years of experience in the fi eld of • Free Phenols spectrophotometric analysis and robot automation • Ortho Phosphate in one design. Advantages are the low ppb level detection limits, high accuracy and the large sample • Silicate capacity. • Sulfate Typical application areas for the BluVision™ are for All our applications conform to regulatory bodies example drinking water, wastewater, ground water such as NEN-ISO 15923-1, CMA/2/I/C.8, EPA, Standard and surface water. Methods for Water and Wastewater (SMWW), ASTM etc. The BluVision™ Discrete analyzer The BluVision™ automates the sample & reagent pipetting into the cuvettes, mixing, heating, blank correction and photometric measurement. The BluVision™ discrete analyzer has 100 sample positions and 32 positions for reagents, (stock) standards and QC’s. The sample and reagent racks are cooled during the analysis run. One needle is used for dispensing sample and reagent into the cuvettes. The needle pre-heats the Autoloader for cuvette blocks samples and reagents prior to dispensing. -

EXPERIMENT 15 TF Notes
EXPERIMENT 15 TF Notes 1. Have students log onto LoggerPro3 and start heating water as soon as they arrive 2. This reaction is easily contaminated so students must never insert the thermometer directly into the cuvette containing the reaction. Instead they should rather measure the temperature of the water and equate that to the temperature of the solution in the cuvette. 3. We will be using hot gloves in this experiment. 4. Make sure you are familiar with the calculations necessary to fill out the chart. All the necessary equations are given at the top fo the page. Note that the temperature must be converted from °C to K. EXPERIMENT 15 Thermodynamics of Complex-Ion Equilibria Introduction Thermodynamic data for a reaction system provides researchers with information that is important from both theoretical and practical points of view. There are several thermodynamic properties that chemists pay close attention to when designing or carrying out experiments such as thermodynamic stability, the change in free energy of a reaction, and temperature dependence. For example, if a chemist wants to create a new type of solar cell that combines a semiconductor material with a novel conductive oxide and wants to make sure that the two materials will not react with each other, thermodynamics provide the answer. By finding the free energy change associated with the reaction, s/he can determine how stable the layers are in contact with each other and to what temperature. In this experiment, you will learn how to determine those parameters from a controlled experiment by using spectrometry to find concentration data at various temperatures. -

A Guide to Colorimetry
A Guide to Colorimetry Sherwood Scientific Ltd 1 The Paddocks Cherry Hinton Road Cambridge CB1 8DH England www.sherwood-scientific.com Tel: +44 (0)1223 243444 Fax: +44 (0)1223 243300 Registered in England and Wales Registration Number 2329039 Reg. Office as above Group I II III IV V VI VII VIII Period 1A 8A 1 2 1 H 2A 3A 4A 5A 6A 7A He 1.008 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.939 9.0122 10.811 12.011 14.007 15.999 18.998 20.183 11 12 13 14 15 16 17 18 3 Na Mg 3B 4B 5B 6B 7B [---------------8B-------------] 1B 2B Al Si P S Cl Ar 22.99 24.312 26.982 28.086 30.974 32.064 35.453 39.948 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.102 40.08 44.956 47.9 50.942 51.996 54.938 55.847 58.933 58.71 63.546 65.37 69.72 72.59 74.922 78.96 79.904 83.8 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 85.47 87.52 88.905 91.22 92.906 95.94 [97] 101.07 102.91 106.4 107.87 112.4 114.82 118.69 121.75 127.6 126.9 131.3 55 56 57* 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Ti Pb Bi Po At Rn 132.91 137.34 138.91 178.49 180.95 183.85 186.2 190.2 192.2 195.09 196.97 200.59 204.37 207.19 208.98 209 210 222 87 88 89** 104 105 106 107 108 109 110 111 112 114 116 7 Fr Ra Ac Rt Db Sg Bh Hs Mt 215 226.03 227.03 [261] [262] [266] [264] [269] [268] [271] [272] [277] [289] [289] 58 59 60 61 62 63 64 65 66 67 68 69 70 71 * Lanthanides Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 140.12 140.91 144.24 145 150.35 151.96 157.25 158.92 152.5 164.93 167.26 168.93 173.04 174.97 90 91 92 93 94 95 96 97 98 99 100 101 102 103 ** Actinides Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr 232.04 231 238 237.05 239.05 241.06 244.06 249.08 251 252.08 257.1 258.1 259.1 262.11 The ability to analyse and quantify colour in aqueous solutions and liquids using a colorimeter is something today’s analyst takes for granted. -

Boeco Kap 2 Laboratory Equipment 2014 Musterseiten Boeco
SPECTROPHOTOMETERS BOECO SPECTROPHOTOMETER MODELS S-200 VIS & S-220 UV/VIS The BOECO S-220 (UV/VIS) and S-200 (VIS) are high quality, compact, low cost measurement systems for daily analysis in LABORATORY EQUIPMENT LABORATORY education, QC and basic research. Compact single beam optics with full range scanning The single beam optics are compact and bench space saving. The long life Hamamatsu Xenon lamp optics in the S-220 ensure quick and reliable performance and the Tungsten Halogen lamp used in S-200 also provide a reliable measurement. Color touch screen operation The intuitive color touch screen operation provides simple access to an extensive range of functions. The touch screen is sensitive to stylus and laboratory gloves. Icon driven on-board software S-200 improves accessibility and the graphical display allows spectrum S-220 or standard curve to be shown on the screen. The forward and back quick key allows the user to proceed or swiftly return to the process. An enlarged data display for photometry measurement makes result reading easier. Various measurement modes Operation modes include photometric, multiple wavelength analysis, spectrum scanning, time scan and kinetics; direct concentration results are included. Optional accessories A variety of accessories are included such as test tube holder, flow cell with sipper, temperature control holder, long path length MAIN MENUE cuvette holder & multiple cell holder are available to enhance different application needs. Storage and data output External storage with SD card and free downloadable PC Software MasterReport (www.boeco.com) allows data export to PC in compatible text or spreadsheet format for further data processing in the PC. -

Ultra-Thin Fluorocarbon Foils Optimise Multiscale Imaging of Three
www.nature.com/scientificreports OPEN Ultra-thin fuorocarbon foils optimise multiscale imaging of three-dimensional native and optically cleared specimens Katharina Hötte1, Michael Koch1, Lotta Hof1, Marcel Tuppi 2,4, Till Moreth1, Monique M. A. Verstegen3, Luc J. W. van der Laan 3, Ernst H. K. Stelzer 1 & Francesco Pampaloni1* In three-dimensional light microscopy, the heterogeneity of the optical density in a specimen ultimately limits the achievable penetration depth and hence the three-dimensional resolution. The most direct approach to reduce aberrations, improve the contrast and achieve an optimal resolution is to minimise the impact of changes of the refractive index along an optical path. Many implementations of light sheet fuorescence microscopy operate with a large chamber flled with an aqueous immersion medium and a further inner container with the specimen embedded in a possibly entirely diferent non-aqueous medium. In order to minimise the impact of the latter on the optical quality of the images, we use multi- facetted cuvettes fabricated from vacuum-formed ultra-thin fuorocarbon (FEP) foils. The ultra-thin FEP-foil cuvettes have a wall thickness of about 10–12 µm. They are impermeable to liquids, but not to gases, inert, durable, mechanically stable and fexible. Importantly, the usually fragile specimen can remain in the same cuvette from seeding to fxation, clearing and observation, without the need to remove or remount it during any of these steps. We confrm the improved imaging performance of ultra-thin FEP-foil cuvettes with excellent quality images of whole organs such us mouse oocytes, of thick tissue sections from mouse brain and kidney as well as of dense pancreas and liver organoid clusters. -

Multiskan Sky Microplate Spectrophotometer Take Your Photometric Research to New Heights Multiskan Sky Microplate Spectrophotometer: Redefining Photometry
Multiskan Sky Microplate Spectrophotometer Take your photometric research to new heights Multiskan Sky Microplate Spectrophotometer: redefining photometry The Thermo Scientific™ Multiskan™ Sky Microplate nucleic acid and protein analyses. It is ideal for multi-user Spectrophotometer is a new-generation instrument environments where a variety of endpoint, kinetic, and designed for the ultimate user experience. By introducing spectral assays are run. Results are provided quickly an attractive user interface that is optimized for and presented conveniently on Multiskan Sky Microplate touch-screen use combined with multiple connectivity Spectrophotometer models with touch screens. These options, we offer refined photometry in life sciences touch-screen models offer multiple connectivity options research and bring unmatched versatility to academic, including unique access to Thermo Fisher Connect biotech, and pharmaceutical laboratories. cloud-based tools. Additionally, the intuitive Thermo Scientific™ SkanIt™ PC Software is powerful enough to Photometric measurement made impeccable address even the most challenging applications. Multiple The Multiskan Sky Microplate Spectrophotometer is languages are available whether the instrument is operated exceptionally convenient and easy to use for virtually via the touch screen or SkanIt Software. any photometric research application, especially Multiskan Sky Microplate Spectrophotometer instruments are available in three different configurations Multiskan Sky Microplate Multiskan Sky Microplate Multiskan Sky Microplate Spectrophotometer Spectrophotometer with Spectrophotometer, with Cuvette and Touch Screen* operated only with SkanIt Touch Screen* PC Software • Offers flexibility to use the • Offers flexibility to use the instrument as stand-alone • Ideal for users who rely instrument as stand-alone or with SkanIt PC software on PC for all operations or with SkanIt PC software • Offers additional cuvette reading functionality * Allows access to Thermo Fisher Connect cloud-based capabilities. -

Spectramax® M2/M2 User Guide
SpectraMax® M2/M2e user guide A MULTI-DETECTION MICROPLATE READER WITH TWO-MODE CUVETTE PORT SpectraMax M2e user guide cover 1 1 4/21/06 9:53:28 AM SpectraMax® M2 SpectraMax® M2e Multimode Plate Readers User Guide Molecular Devices Corporation 1311 Orleans Drive Sunnyvale, California 94089 Part #0112-0102 Rev. D. SpectraMax M2 & SpectraMax M2e Multi-mode Plate Readers User Guide — 0112-0102 Rev. D Copyright © Copyright 2006, Molecular Devices Corporation. All rights reserved. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into any language or computer language, in any form or by any means, electronic, mechanical, magnetic, optical, chemical, manual, or otherwise, without the prior written permission of Molecular Devices Corporation, 1311 Orleans Drive, Sunnyvale, California, 94089, United States of America. Patents The SpectraMax M2 and SpectraMax M2e and use thereof are covered by issued U.S. Patent nos. 5,112,134; 5,766,875; 5,959,738; 6,188,476; 6,232,608; 6,236,456; 6,313,471; 6,316,774; 6,320,662; 6,339,472; 6,404,501; 6,496,260; and foreign patents. Other U.S. and foreign patents pending. Trademarks PathCheck, SpectraMax and SoftMax are registered trademarks and Automix is a trademark of Molecular Devices Corporation. All other company and product names are trademarks of their respective owners. Disclaimer Molecular Devices Corporation reserves the right to change its products and services at any time to incorporate technological developments. This user guide is subject to change without notice. Although this user guide has been prepared with every precaution to ensure accuracy, Molecular Devices Corporation assumes no liability for any errors or omissions, nor for any damages resulting from the application or use of this information. -

Spectrophotometer Health & Safety Document Including General
Spectrophotometer Health & Safety Document including General Operating Instructions Original Instructions Page finder 1. Health and Safety 3 1.1. Scope 3 1.2. Intended use 3 1.3. Safety Notices 3 1.4. General Safety 4 1.5. General Hazards 5 1.6. Unpacking and Installation 5 2. Operating and Maintenance 7 2.1. Preparation before starting 7 2.2. Performing a run - cuvettes 7 2.3. Performing a run – Micro Volume Instruments 8 2.4. Post run procedures 9 2.5. User Maintenance 9 2.6. Novaspec III/Plus Lamp Change 10 2.7. Ultrospec 8000/9000 Lamp Change 10 3. Troubleshooting 11 3.1. Emergency Procedures 12 3.2. Recycling Procedures 12 3.3. Recycling of hazardous substances 12 3.4. Disposal of electrical components 12 3.5. Specifications 13 3.6. Manufacturing information 13 4. Legal 14 29-0047-78 AC 04-2014 2 1. HEALTH AND SAFETY 1.1. Scope This booklet covers the following ranges of instruments: • Ultrospec™ 10 cell density meter • Novaspec™ III and Novaspec Plus Visible spectrophotometers • GeneQuant™ 100 & 1300 UV/Visible spectrophotometers • Ultrospec 2100/7000/8000/9000 UV/Visible spectrophotometers • SimpliNano™ and NanoVue™ Plus micro volume spectrophotometer Translations of these instructions into 22 European languages are available on the User Manual CD supplied with the instrument and on the internet at GE home page www.gelifesciences.com 1.2. Intended use Visible and UV/Visible spectrophotometers shine light through a liquid sample and measure its Absorbance. This sample is typically held in a cuvette but when sample volume is limited, instruments such as the SimpliNano or NanoVue Plus can be used where a 2 µl sample can be pipetted directly onto the measurement area. -

Transforming Plasmid DNA Into Electrocompetent Cells
Transforming plasmid DNA into electrocompetent cells 1. Clean and dry electroporation cuvettes throroughly on the cuvette washer. Chill on ice and allow to air dry. Use one cuvette for each DNA sample you are transforming. 2. Dialyze your DNA sample(s) using a nitrocellulose filter and DI water. • Fill a Petri dish with DI water. • Place a single nitrocellulose filter paper on the surface of the water – shiny side up. If you are dialyzing more than one sample, it helps to cut a small notch in the paper to serve as a “key”. This helps to identify/keep track of your samples. • Spot DNA samples onto the surface of the paper. Space out the samples a little bit because they will expand in volume as they dialyze. • Dialyze your sample for 10-15 minutes. *This step is particularly important if the DNA has undergone any previous manipulations that have introduced salts (adding buffers for ligation, restriction enzyme digestions, etc.). Too much salt in the DNA will cause your sample to arc when electroporating. This will kill the cells and significantly reduce the efficiency of transformation. If your sample arcs, it may still be worth while to plate your cells. However, it is likely that you will get very few (if any) colonies. You should repeat this sample. Main factors that can cause arcing - too much salt in your DNA, water on the outside of the cuvette, oil on the outside of the cuvette from handling it too much without gloves, too much salt in the cells. 3. Thaw electrocompetent cells on ice. -

AMSA Meat Color Measurement Guidelines
AMSA Meat Color Measurement Guidelines Revised December 2012 American Meat Science Association http://www.m eatscience.org AMSA Meat Color Measurement Guidelines Revised December 2012 American Meat Science Association 201 West Springfi eld Avenue, Suite 1202 Champaign, Illinois USA 61820 800-517-2672 [email protected] http://www.m eatscience.org iii CONTENTS Technical Writing Committee .................................................................................................................... v Preface ..............................................................................................................................................................vi Section I: Introduction ................................................................................................................................. 1 Section II: Myoglobin Chemistry ............................................................................................................... 3 A. Fundamental Myoglobin Chemistry ................................................................................................................ 3 B. Dynamics of Myoglobin Redox Form Interconversions ........................................................................... 3 C. Visual, Practical Meat Color Versus Actual Pigment Chemistry ........................................................... 5 D. Factors Affecting Meat Color ............................................................................................................................... 6 E. Muscle