11 the Functional Role of Soil Seed Banks in Natural Communities
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Deep Now & the Seed Bank Project
DEEP NOW & THE SEED BANK PROJECT by Rachael Marne Jones A thesis submitted in partial fulfillment Of the requirements for the degree of Masters of Fine Arts in Art MONTANA STATE UNIVERSITY Bozeman, Montana April 2018 ©COPYRIGHT by Rachael Marne Jones 2018 All Rights Reserved ii TABLE OF CONTENTS 1. INTRODUCTION ...........................................................................................................1 2. BODY ..............................................................................................................................3 3. CONCLUSION ..............................................................................................................30 REFERENCES CITED ......................................................................................................32 iii LIST OF FIGURES Figure Page 1. Detail of Landmark II ..........................................................................................6 2. Detail of Out of Site .............................................................................................9 3. Flow State Diptych .............................................................................................12 4. Instructional Drawing For The Seed Bank Project: Step 1 ...............................13 5. Instructional Drawing For The Seed Bank Project: Step 2 ...............................13 6. Ana Mendietta. Four stills from Silhueta Sangrieta ..........................................17 7. Prototype For The Long Now Clock .................................................................19 -

Estimating Soil Seed Bank Characteristics in Ponderosa Pine Forests Using Vegetation and Forest-Floor Data
Estimating Soil Seed Bank Characteristics in Ponderosa Pine Forests Using United States Department of Agriculture Vegetation and Forest-Floor Data Forest Service Rocky Mountain Research Station Research Note Scott R. Abella and Judith D. Springer RMRS-RN-35 September 2008 Abstract—Soil seed banks are important for vegetation management because they contain propagules of species that may be considered desirable or undesirable for site colonization after management and disturbance events. Knowledge of seed bank size and composition before planning management activities facilitates proactive management by providing early alerts of exotic species presence and of abilities of seed banks to promote colonization by desirable species. We developed models in ponderosa pine (Pinus ponderosa) forests in northern Arizona to estimate the size and richness of mineral soil seed banks using readily observable vegetation and forest- floor characteristics. Regression models using three or fewer predictors explained 41 to 59 percent of the variance in 0- to 2-inch (0- to 5-cm) seed densities of total and native perennial seed banks. Key predictors included aboveground plant species richness/10.8 ft2 (1 m2), litter weight and thickness, and tree canopy type (open or closed). Both total and native perennial seed banks were larger and richer in plots containing: (1) species-rich understories, (2) sparse litter, and (3) tree canopy openings. A regression tree model estimated that seed bank density of native perennials is 14-fold greater if aboveground plant richness exceeds eight species/10.8 ft2, forest-floor leaf litter is < 1 inch (2.5 cm) thick, and tree canopies are open. Introduction Soil seed banks are important for vegetation man- agement in ponderosa pine forests in at least four ways. -
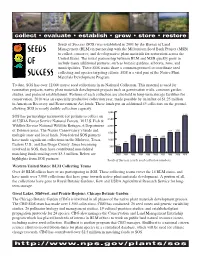
BLM Seeds of Success Program
collect • evaluate • establish • grow • store • restore Seeds of Success (SOS) was established in 2001 by the Bureau of Land Management (BLM) in partnership with the Millennium Seed Bank Project (MSB) to collect, conserve, and develop native plant materials for restoration across the United States. The initial partnership between BLM and MSB quickly grew to include many additional partners, such as botanic gardens, arboreta, zoos, and municipalities. These SOS teams share a common protocol to coordinate seed collecting and species targeting efforts. SOS is a vital part of the Native Plant Materials Development Program. To date, SOS has over 12,000 native seed collections in its National Collection. This material is used for restoration projects, native plant materials development projects such as germination trials, common garden studies, and protocol establishment. Portions of each collection are also held in long-term storage facilities for conservation. 2010 was an especially productive collection year, made possible by an influx of $1.25 million in American Recovery and Reinvestment Act funds. These funds put an additional 43 collectors on the ground, allowing SOS to nearly double collection capacity. 3,000 ~2,700 SOS has partnerships nationwide for permits to collect on 40 USDA Forest Service National Forests, 10 U.S. Fish & 2,500 Wildlife Service National Wildlife Refuges, 6 Department 2,000 of Defense areas, The Nature Conservancy’s lands and 1,474 1,504 1,500 multiple state and local lands. Non-federal SOS partners 1,235 1,208 1,012 1,044 1,058 have made significant collections in the Midwest, Texas, 1,000 685 447 Eastern U.S., and San Diego County. -

Seeds & Seed Practices
A Preliminary Report on Seeds & Seed Practices across the United States For too many people and communities around the world, patenting seeds and the genetic manipulation of seeds the dominant agricultural model is causing economic for corporate profit. We must shed light on and uphold hardship, the destruction of biological diversity, and the the rights of all of life to grow, evolve, and be present in exploitation of earth’s ecological commons. It is a model ecology, even the whole of the Earth. Seeds are one of the based on the commodification of life. We can no longer foundational elements to our food system and must be continue the status quo that enables multi-national defended because they affirm our lives, our very suste- corporations to corner our food system and our seed nance, and our relationship to place, culture, plants, and commons. Every element that is foundational to life (food, all of nature. water, land, air) is under threat of privatization and mar- ketization by an economic order that seeks to profit and This report is written to examine current seed culture, sav- own our common wealth. ing, keeping and sharing, and seed advocacy across the United States and North America. This is an initial report The growing global movement by peasants, growers, of the seed survey sent out by the Rights of the Mother farm workers, fisherfolk, pastoralists, indigenous peoples, Earth/Defense of the Commons Workgroup of the U.S. urban growers, food system policy advocates, and human Food Sovereignty Alliance in summer 2013, to deepen the rights activists has born witness to a growing trend of discussion around seeds and the rights of nature. -

Plant Genetic Resources
12b. Genetic resources for food and agriculture: plant genetic resources Type: State / Benefits Indicator Indicator Description Seed banks provide an insurance policy against the extinction of plants in the wild. They complement in situ conservation methods, which conserve plants and animals directly in the wild. The indicator is based on an enrichment Index developed by the United Nations Food and Agriculture Organisation (FAO, 2010a&b) to assess the genetic diversity held in gene banks. The method factors in duplication and similarity to existing accessions. An upward trending line indicates diversity is being added to collections – the steeper the line, the greater the diversity being incorporated. An accession is a collection of plant material from a particular location at a point in time. No update since previous publication. As a result of discussions in the UK Plant Genetic Resources Group, a revised indicator is being considered; whilst development is underway it is not ready for publication. UK Enrichment Index There was a 15% increase in the Enrichment Index between 2013 and 2018. A rapid rise in the Enrichment Index since 2000 can be attributed to a concerted collection effort by the Millennium Seed Bank. There is considerable annual variability in the number of new accessions into UK germplasm collections. The total number of accessions has risen since 1960, totalling 93,786 accessions by June 2018. Figure 12b.1: Cumulative Enrichment Index of plant genetic resource collections held in the UK and annual number of accessions into UK germplasm collections, 1960 to 2018 Notes: 1. Data was obtained from EURISCO, which collates information across Europe from national germplasm collections, including the UK National Inventory of Plant Genetic Resources. -

Soil Seed Bank Dynamics in Transferred Topsoil
SOIL SEED BANK DYNAMICS IN TRANSFERRED TOPSOIL EVALUATING RESTORATION POTENTIALS William M. Fowler Bachelor of Science in Environmental Science and Environmental Restoration School of Environmental Science October 2012 Acknowledgements Thanks to my supervisors Dr. Joe Fontaine and Prof. Neal Enright for all the advice and support given over the last year. I would also like to thank the members of the Murdoch University, Terrestrial Ecology Research Group (TERG) for their valuable assistance and guidance, especially Willa Veber and Dr. Philip Ladd, as well as the Murdoch University Environmental Science Association (MUEnSA) for helping find enthusiastic volunteer field assistants. I wish to thank the Department of Environment and Conservation (DEC) for providing technical and financial assistance in addition to information and resources that were vital to this projects success. Finally I would like to thank the Environmental Weeds Action Network (EWAN) for awarding me a scholarship to help me complete this valuable research. DECLARATION I declare that this thesis is my own original work and has not been submitted for any other unit for academic credit. William Michael Fowler Soil seed bank dynamics of transferred topsoil: evaluating restoration potentials ABSTRACT Global change, increasing human population growth and urbanisation represent increasing pressures on biodiversity and ecosystem function. It is now widely recognised that conservation of existing natural fragments will not be sufficient to maintain extant biodiversity or meet conservation goals. Thus there is a major and rapidly expanding need for the practice of ecological restoration whereby degraded lands are managed to increase and maintain indigenous species. A soil seed bank germination experiment was conducted over a period of 13 weeks. -

Gene Banks Pay Big Dividends to Agriculture, the Environment, and Human Welfare R
Community Page Gene Banks Pay Big Dividends to Agriculture, the Environment, and Human Welfare R. C. Johnson early a century after the pioneering American Napple tree purveyor Johnny Appleseed traveled from town to town planting nurseries in the Midwestern United States, Frans Nicholas Meijer left his Netherlands home to pursue a similar vocation as an “agricultural explorer” for the US Department of Agriculture. Over the course of his career, Meijer, who changed his name to Frank Meyer after reaching the New World, helped introduce over 2,500 foreign plants from Europe, Russia, and China, including the lemon that would bear his name. Starting with his first expedition for Asian plants in 1905, Meyer would encounter isolation, physical discomfort, disease, robbers, and revolutionaries in his quest to collect useful plants. Although some of Meyer’s collections are still used today, only a few are conserved in their original doi:10.1371/journal.pbio.0060148.g001 form. They, along with countless other Figure 1. Collecting Accessions collections from the early 20th century, Walter Kaiser collects taper-tip onion (Allium acuminatum) along the Snake River in Idaho in 2005. disappeared because there was no long- Collections were made across a broad area of Western rangeland to strengthen the WRPIS Allium term system for conservation. To rectify collection and for research to determine taper-tip onion adaptation zones needed for successful revegetation. this problem, Congress established a system of repositories after World and maintain germplasm is ongoing genetic uniformity and dependence on War II to maintain and distribute and urgent. Since agriculture’s just a few crops. -

Activity 1: Seed Collection and Observation
Activity 1: Seed Collection and Observation In this activity, learners will extract seeds from produce in your garden or fridge for observation and analysis. They will collect data about the seeds and create a seed-bank art piece to prepare for space. *Background information can be found at the end of this activity guide* Activity Suggested Materials In order to grow food in space, there is one thing astronauts will desperately need: seeds! Almost all plants come from seeds, which can be grown to provide food. Food production is an essential key to long- Data Sheet (printable term survival on another planet. In order to investigate how humans version) might grow food in space, our project begins with an observation and 3-6 different fruits or analysis of seeds! vegetables with seeds Knife and spoon 1. Gather your learners and the materials needed! Cutting board or surface 2. Begin with a discussion, using some of the questions below: to place seeds on a. Why are seeds important for plants? Writing utensils b. How might seeds be similar or different from plant to Ruler plant? Scale (optional) 3. Gather 3-6 different fruits or vegetables from around the house Magnifying glass or outside. Produce items, like apples, oranges, and squash work (optional) best for this activity, but feel free to expand beyond that. Glue 4. Record the items on your data sheet and make a quick Craft Paper hypothesis: Markers, crayons, or a. What do you think the seeds will be like from each plant? colored pencils (optional) 5. With adult help, use a knife to cut open each item. -

Samara English Edition 34 (PDF)
The International Newsletter of the Millennium Seed Bank Partnership July - December 2018 brahmsonline.kew.org/msbp/Training/Samara ISSN 1475-8245 Issue: 34 PuttingPutting seedsseeds toto goodgood useuse KyrgyzstanKyrgyzstan exploresexplores thethe pharmacologicalpharmacological potentialpotential ofof itsits nativenative floraflora Landscape of Kyrgyzstan Photo: RBG Kew ANARA UMRALINA (Head of Plant Biotechnology Laboratory, Institute of Biotechnology National Academy of Sciences of the Kyrgyz Republic) Contents Page 1. Putting seeds to good use. SERGEY HEGAY Page 3. A message from Jonas Mueller. A message from Sandrine Godefroid. (Plant Biotechnology Researcher, Biotechnology Institute National Academy of Scien- Page 4. Reintroduction of endangered grassland species in ces of Kyrgyz Republic) Luxembourg. Page 5. RBG Kew develops a land restoration model to support the Great Green Wall in sub-Saharan Africa. yrgyzstan, despite its rather limited territory, has one of the richest floras in Cen- Page 6. Achieving GSPC Target 8 in Azerbaijan. tral Asia, with around 4,000 species described to date, at least 10% of which are Page 7. Seed Banking as a Last Resort for Endangered endemic or sub-endemic (i.e. grow only in Kyrgyzstan or slightly beyond its bor- Plant Populations. K Young researchers experience the Austrian Alps ders). The floristic diversity is partly due to Kyrgyzstan’s geographic location in the heart through seeds. of the mountain systems of Tian Shan and Pamir Alay, where the vegetation ranges from Page 8-9.Germination research and species reintroductions. semi-desert to tall herb meadows and from fruit and nut forests to alpine pastures. Page 10.A model of reforestation, food security and long-term carbon sequestration in Haiti. -

Mechanisms Underpinning the Onset of Seed Coat Impermeability And
www.nature.com/scientificreports OPEN Mechanisms underpinning the onset of seed coat impermeability and dormancy-break in Astragalus Received: 13 November 2018 Accepted: 24 June 2019 adsurgens Published: xx xx xxxx Ganesh K. Jaganathan1, Jiajin Li1, Matthew Biddick2, Kang Han1, Danping Song1, Yashu Yang1, Yingying Han1 & Baolin Liu1 Impermeable seed coats, i.e. physical dormancy (PY) infuence the germination ecology of plants from 18 angiosperm families. Astragalus adsurgens (Fabaceae; Papilinoidaae) is a perennial plant widespread in temperate regions that is thought to produce both permeable and impermeable seeds. Why seeds vary in the permeability of their coat, in addition to the mechanisms by which impermeable seeds break dormancy, are not completely understood. However, seeds are often consumed by herbivores; a phenomenon that might facilitate the germination of impermeable seeds. Here, we tested whether: (1) moisture content plays a signifcant role in the onset of seed coat impermeability (and therefore PY) at similar ranges reported for species from tropical ecosystems; and (2) the presence of impermeable coats ofer any benefts for seed survival when consumed by animals. We tested these hypotheses using A. adsurgens seeds collected from Inner Mongolia, China. Freshly collected seeds with a moisture content of 9.7% were permeable to water and therefore not physically dormant. However, seeds became impermeable when dried below a threshold of 6.5% moisture content. Treating impermeable seeds with hydrochloric acid efectively broke dormancy. Scanning electron microscope (SEM) revealed that HCl treated seeds had a narrow opening in the hilum and extra-hilar regions, through which water entered. Seeds with impermeable coats survived signifcantly better than permeable seeds when consumed by cows. -

Direct and Indirect Effects of Temperature and Precipitation on Alpine Seed Banks in the Tibetan Plateau
Ecological Applications, 30(5), 2020, e02096 © 2020 by the Ecological Society of America Direct and indirect effects of temperature and precipitation on alpine seed banks in the Tibetan Plateau 1,3 2 1 MIAOJUN MA, SCOTT L. COLLINS , AND GUOZHEN DU 1State Key Laboratory of Grassland and Agro-ecosystems, School of Life Sciences, Lanzhou University, Lanzhou 730000 China 2Department of Biology, University of New Mexico, Albuquerque, New Mexico 87103 USA Citation: Ma, M., S. L. Collins, and G. Du. 2020. Direct and indirect effects of temperature and precipita- tion on alpine seed banks in the Tibetan Plateau. Ecological Applications 30(5):e02096. 10.1002/eap.2096 Abstract. Plant community responses to global environmental change focus primarily on aboveground vegetation; however, the important role of the seed bank is frequently neglected. Specifically, the direct and indirect effects of changes in temperature and precipitation on seed banks remain poorly understood, yet seed banks provide a vital source of ecosystem resilience to global environmental change. We used a structural equation model to explore the direct and indirect effects of temperature, precipitation, and other biotic and abiotic factors on soil seed bank community composition using 1,026 soil seed bank samples from 57 sites along an eleva- tion gradient that served as a space-for-time substitution for changing climate in the Tibetan Plateau. Seed bank richness was negatively correlated with both precipitation and temperature, but neither climate factor affected seed bank density. Temperature was also negatively corre- lated with vegetation species richness, which was positively correlated with seed bank richness and density. Both precipitation and temperature were positively correlated with soil total N, and total N was negatively correlated with vegetation richness. -

Seed Bank Response to Prescribed Fire in the Central Appalachians
United States Department of Agriculture Seed Bank Response to Forest Service Prescribed Fire in the Northern Research Station Research Paper NRS-9 Central Appalachians Thomas M. Schuler Melissa Thomas Van-Gundy Mary B. Adams W. Mark Ford Abstract We compared pre- and post-treatment seed bank characteristics of woody species after two prescribed fires in a mesic mixed-oak forest in the central Appalachians. Nineteen woody species were identified from soil samples. Mean species richness and diversity declined but evenness did not. The seed bank was dominated by black birch (Betula lenta), yellow-poplar (Liriodendron tulipifera), blackberry (Rubus spp.), grapevine (Vitis spp.), and Hercules club (Aralia spinosa) before burning. Following burning, the median density of seed bank propagules declined by 45 percent (247.6 to 138.1 m-2). Black birch, yellow-poplar, and grapevine declined by 69, 56, and 40 percent, respectively. The results illustrate the importance of the seed bank as a robust source of non-oak regeneration in this forest type and of the potential effect of fire in altering it. Oak restoration efforts that do not account for this significant source of regeneration in mesic mixed-oak forests likely will be unsuccessful. The Authors THOMAS M. SCHULER, research forester, is currently engaged in research on the ecology and silviculture of Appalachian hardwood forests and the restoration of central Appalachian spruce forests at the Northern Research Station’s Timber and Watershed Laboratory in Parsons, West Virginia. MELISSA THOMAS-VAN GUNDY, research forester, joined the Northern Research Station’s Timber and Watershed Laboratory at Parsons, West Virginia in 2006 and is engaged in research on management and ecology of Appalachian forests.