The Pericyte Microenvironment During Vascular Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Threatening External Iliac Vein Injury During Total Hip Arthroplasty
경희의학 : 제 27 권 제 1 호 □ 증 례 □ J Kyung Hee Univ Med Cent : Vol. 27, No. 1, 2011 Threatening External Iliac Vein Injury during Total Hip Arthroplasty Kang Woo Lee, M.D., Jo Young Hyun, M.D., Jae Woo Yi, M.D., Ph.D. Department of Anesthesiology and Pain Medicine, School of Medicine, Kyung Hee University, Seoul, Korea INTRODUCTION nal iliac vein during total hip replacement, which resulted in hemorrhagic shock. Since its inception in the 1960’s, total hip arthro- plasty (THA) has grown in volume and complexity. CASE REPORT Total hip arthroplasty has revolutionized the treatment of end-stage hip arthritis and is felt to be among the A 63-year-old female patient (150 cm, 59 kg) was most cost effective of all medical interventions diagnosed as secondary osteoarthritis of left hip joint. available.(1) The complications associated with THA She was scheduled to undergo total hip replacement. have been increased as a result of increasing life The patient’s previous medical history entailed ten expectancy and widened indications for THA. But the years of hypertension, which was well-controlled with complications related to total hip arthroplasty are not oral anti-hypertensive drugs. Routine monitors such as common. Especially vascular injuries are very rare but electrocardiogram, pulse oxymeter, non-invasive blood can cause limb loss or become life threatening. A pressure were used. Induction of anesthesia was achieved thorough understanding of the possible complications with propofol 120 mg, rocuronium 50 mg intravenously. following total hip arthroplasty aids in optimizing Anesthesia was maintained with sevoflurane. After loss patient outcomes The occurrence of the complication of all four twitches from the train-of-four obtained by related to THA has not been emphasized enough in ulnar nerve stimulation, endotracheal intubation was anesthesia literature and has been overlooked in the performed. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -

Arteries to Arterioles
• arteries to arterioles Important: The highest pressure of circulating blood is found in arteries, and gradu- ally drops as the blood flows through the arterioles, capillaries, venules, and veins (where it is the lowest). The greatest drop in blood pressure occurs at the transition from arteries to arterioles. Arterioles are one of the blood vessels of the smallest branch of the arterial circula- tion. Blood flowing from the heart is pumped by the left ventricle to the aorta (largest artery), which in turn branches into smaller arteries and finally into arterioles. The blood continues to flow through these arterioles into capillaries, venules, and finally veins, which return the blood to the heart. Arterioles have a very small diameter (<0.5 mm), a small lumen, and a relatively thick tunica media that is composed almost entirely of smooth muscle, with little elastic tissue. This smooth muscle constricts and dilates in response to neurochemical stimuli, which in turn changes the diameter of the arterioles. This causes profound and rapid changes in peripheral resistance. This change in diameter of the arteri- oles regulates the flow of blood into the capillaries. Note: By affecting peripheral resistance, arterioles directly affect arterial blood pressure. Primary function of each type of blood vessel: - Arteries - transport blood away from the heart, generally have blood that is rich in oxygen - Arterioles - control blood pressure - Capillaries - diffusion of nutrients/oxygen - Veins - carry blood back to the heart, generally have blood that is low in oxygen. -

Artery/Vein Classification of Blood Vessel Tree in Retinal Imaging
Artery/vein Classification of Blood Vessel Tree in Retinal Imaging Joaquim de Moura1, Jorge Novo1, Marcos Ortega1, Noelia Barreira1 and Pablo Charlon´ 2 1Departamento de Computacion,´ Universidade da Coruna,˜ A Coruna,˜ Spain 2Instituto Oftalmologico´ Victoria de Rojas, A Coruna,˜ Spain joaquim.demoura, jnovo, mortega, nbarreira @udc.es, [email protected] { } Keywords: Retinal Imaging, Vascular Tree, Segmentation, Artery/vein Classification. Abstract: Alterations in the retinal microcirculation are signs of relevant diseases such as hypertension, arteriosclerosis, or diabetes. Specifically, arterial constriction and narrowing were associated with early stages of hypertension. Moreover, retinal vasculature abnormalities may be useful indicators for cerebrovascular and cardiovascular diseases. The Arterio-Venous Ratio (AVR), that measures the relation between arteries and veins, is one of the most referenced ways of quantifying the changes in the retinal vessel tree. Since these alterations affect differently arteries and veins, a precise characterization of both types of vessels is a key issue in the development of automatic diagnosis systems. In this work, we propose a methodology for the automatic vessel classification between arteries and veins in eye fundus images. The proposal was tested and validated with 19 near-infrared reflectance retinographies. The methodology provided satisfactory results, in a complex domain as is the retinal vessel tree identification and classification. 1 INTRODUCTION Hence, direct analysis of many injuries caused by oc- ular pathologies can be achieved, as is the case, for The analysis of the eye fundus offers useful infor- example, the diabetic retinopathy (DR). The DR is a mation about the status of the different structures the diabetes mellitus complication, one of the principal human visual system integrates, as happens with the causes of blindness in the world (Pascolini, 2011). -

Dual Role of Pericyte Α6β1-Integrin in Tumour Blood Vessels Louise E
© 2017. Published by The Company of Biologists Ltd | Journal of Cell Science (2017) 130, 1583-1595 doi:10.1242/jcs.197848 RESEARCH ARTICLE Dual role of pericyte α6β1-integrin in tumour blood vessels Louise E. Reynolds1,‡, Gabriela D’Amico1,*, Tanguy Lechertier1,*, Alexandros Papachristodoulou2, JoséM. Muñoz-Félix1, Adelè De Arcangelis3, Marianne Baker1, Bryan Serrels4 and Kairbaan M. Hodivala-Dilke1 ABSTRACT preclinical studies (Yamada et al., 2006; Tabatabai et al., 2010) but The α6β1-integrin is a major laminin receptor, and formation of a has had limited success in clinical trials (Patel et al., 2001), therefore laminin-rich basement membrane is a key feature in tumour blood new targets, including pericytes are currently being examined. vessel stabilisation and pericyte recruitment, processes that are Tumour blood vessels have many structural abnormalities important in the growth and maturation of tumour blood vessels. including decreased endothelial barrier function, reduced pericyte However, the role of pericyte α6β1-integrin in angiogenesis is largely recruitment and poor basement membrane organisation when unknown. We developed mice where the α6-integrin subunit is compared with normal quiescent vessels (Armulik et al., 2005). deleted in pericytes and examined tumour angiogenesis and growth. Studies have shown that pericyte recruitment and investment to These mice had: (1) reduced pericyte coverage of tumour blood blood vessels stimulates endothelial cell basement membrane (BM) vessels; (2) reduced tumour blood vessel stability; (3) increased deposition and organisation in vitro (Stratman et al., 2009). This is blood vessel diameter; (4) enhanced blood vessel leakiness, and mediated mainly by secretion of endothelial platelet-derived growth β β (5) abnormal blood vessel basement membrane architecture. -

Major Blood Vessel Reconstruction During Sarcoma Surgery
PAPER Major Blood Vessel Reconstruction During Sarcoma Surgery Tae K. Song, MD; E. John Harris Jr, MD; Shyam Raghavan, BS; Jeffrey A. Norton, MD Objective: To evaluate the outcomes of major vessel re- (7 high grade and 6 low grade) and 1, benign (leio- construction as part of surgery to remove sarcomas. myoma). Seven patients had replacement of artery and vein; 5, artery only; and 2, vein only. In all, 16 arteries Design: Retrospective review. were reconstructed (2 common femoral; 5 iliac; 2 super- ficial femoral; 1 brachial; 1 popliteal; and 2 aorta, one with Setting: Tertiary academic medical center. implantation of both iliac arteries and the other with im- plantation of the left renal, superior mesenteric, and he- Patients: Fourteen patients (10 female) with retroperi- patic arteries). Eight patients (57%) had 9 veins recon- toneal or extremity sarcomas and major blood vessel in- structed (3 external iliac, 3 superficial femoral, 2 vena volvement who underwent surgery to remove the tu- cava, and 1 popliteal). Primary arterial patency was 58% mor and had blood vessel reconstruction between 2003 and primary-assisted patency was 83%. Venous patency and 2008. Each patient underwent computed tomogra- was 78%. Local recurrence occurred in 3 patients (21%). phy angiography. Five-year disease-free and overall survival were 52% and 68%, respectively. Limb salvage was achieved in 93%. Main Outcome Measures: Early (Ͻ30 days) and late (Ͼ30 days) operative morbidity and mortality, freedom Conclusion: Involvement of vascular structures is not from disease, and graft patency. a contraindication for resection of sarcomas, but appro- priate planning is necessary to optimize outcome. -

Anatomy Review: Blood Vessel Structure & Function
Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.awl.com/bc) Page 1. Introduction • The blood vessels of the body form a closed delivery system that begins and ends at the heart. Page 2. Goals • To describe the general structure of blood vessel walls. • To compare and contrast the types of blood vessels. • To relate the blood pressure in the various parts of the vascular system to differences in blood vessel structure. Page 3. General Structure of Blood Vessel Walls • All blood vessels, except the very smallest, have three distinct layers or tunics. The tunics surround the central blood-containing space - the lumen. 1. Tunica Intima (Tunica Interna) - The innermost tunic. It is in intimate contact with the blood i n the lumen. It includes the endothelium that lines the lumen of all vessels, forming a smooth, friction reducing lining. 2. Tunica Media - The middle layer. Consists mostly of circularly-arranged smooth muscle cells and sheets of elastin. The muscle cells contract and relax, whereas the elastin allows vessels to stretch and recoil. 3. Tunica Adventitia (Tunica Externa) - The outermost layer. Composed of loosely woven collagen fibers that protect the blood vessel and anchor it to surrounding structures. Page 4. Comparison of Arteries, Capillaries, and Veins • Let's compare and contrast the three types of blood vessels: arteries, capillaries, and veins. • Label the artery, capillary and vein. Also label the layers of each. Interactive Physiology • Arteries are vessels that transport blood away from the heart. -

Blood Vessels and Circulation
19 Blood Vessels and Circulation Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Functional Anatomy of Blood Vessels Learning Outcomes 19.1 Distinguish between the pulmonary and systemic circuits, and identify afferent and efferent blood vessels. 19.2 Distinguish among the types of blood vessels on the basis of their structure and function. 19.3 Describe the structures of capillaries and their functions in the exchange of dissolved materials between blood and interstitial fluid. 19.4 Describe the venous system, and indicate the distribution of blood within the cardiovascular system. © 2018 Pearson Education, Inc. Module 19.1: The heart pumps blood, in sequence, through the arteries, capillaries, and veins of the pulmonary and systemic circuits Blood vessels . Blood vessels conduct blood between the heart and peripheral tissues . Arteries (carry blood away from the heart) • Also called efferent vessels . Veins (carry blood to the heart) • Also called afferent vessels . Capillaries (exchange substances between blood and tissues) • Interconnect smallest arteries and smallest veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Two circuits 1. Pulmonary circuit • To and from gas exchange surfaces in the lungs 2. Systemic circuit • To and from rest of body © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits 1. Right atrium (entry chamber) • Collects blood from systemic circuit • To right ventricle to pulmonary circuit 2. Pulmonary circuit • Pulmonary arteries to pulmonary capillaries to pulmonary veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits (continued) 3. Left atrium • Receives blood from pulmonary circuit • To left ventricle to systemic circuit 4. -

Hemodynamic Changes in the Carotid Artery After Infusion of Normal Saline Using Computational Fluid Dynamics
diagnostics Article Hemodynamic Changes in the Carotid Artery after Infusion of Normal Saline Using Computational Fluid Dynamics 1, 1, 2 1,3, 2, Ui Yun Lee y, Chul In Kim y, Gyung Ho Chung , Jinmu Jung * and Hyo Sung Kwak * 1 Division of Mechanical Design Engineering, Jeonbuk National University, Jeonju 54896, Korea; [email protected] (U.Y.L.); [email protected] (C.I.K.) 2 Department of Radiology and Research Institute of Clinical Medicine of Jeonbuk National University, Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju 54907, Korea; [email protected] 3 Hemorheology Research Institute, Jeonbuk National University, Jeonju 54896, Korea * Correspondence: [email protected] (J.J.); [email protected] (H.S.K.); Tel.: +82-63-270-4572 (J.J.); +82-63-250-2582 (H.S.K.); Fax: +82-63-270-2460 (J.J.); +82-63-272-0481 (H.S.K.) Co-first authors: Ui Yun Lee and Chul In Kim. y Received: 26 May 2020; Accepted: 9 July 2020; Published: 12 July 2020 Abstract: Purpose: To study the effect of the infusion of normal saline on hemodynamic changes in healthy volunteers using computational fluid dynamics (CFD) simulation. Methods: Eight healthy subjects participated and 16 carotid arteries were used for the CFD analysis. A one-liter intravenous infusion of normal saline was applied to the participants to observe the hemodynamic variations. Blood viscosity was measured before and after the injection of normal saline to apply the blood properties on the CFD modeling. Blood viscosity, shear rate, and wall shear stress were visually and quantitatively shown for the comparison between before and after the infusion of normal saline. -
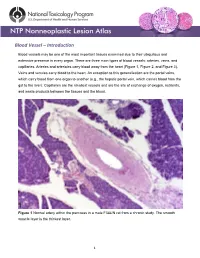
Blood Vessel – Introduction
Blood Vessel – Introduction Blood vessels may be one of the most important tissues examined due to their ubiquitous and extensive presence in every organ. There are three main types of blood vessels: arteries, veins, and capillaries. Arteries and arterioles carry blood away from the heart (Figure 1, Figure 2, and Figure 3). Veins and venules carry blood to the heart. An exception to this generalization are the portal veins, which carry blood from one organ to another (e.g., the hepatic portal vein, which carries blood from the gut to the liver). Capillaries are the smallest vessels and are the site of exchange of oxygen, nutrients, and waste products between the tissues and the blood. Figure 1 Normal artery within the pancreas in a male F344/N rat from a chronic study. The smooth muscle layer is the thickest layer. 1 Blood Vessel – Introduction Figure 2 Normal tunica media (asterisks) and tunica intima (arrows) of an artery within the pancreas in a male F344/N rat from a chronic study (higher magnification of Figure 1). Arteries and veins have walls composed of three layers: the tunica intima, the tunica media, and the tunica adventitia. The tunica intima is composed of endothelial cells on a basement membrane and a subendothelial layer of collagen and elastic fibers. The tunica media consists of smooth muscle cells, elastic fibers, and collagen. The tunica adventitia is composed of collagen and elastic fibers. The relative amounts of each of these components vary depending on the type of vessel. The tunica intima and tunica media of elastic arteries are generally thicker than those of the other types, and the tunica 2 Blood Vessel – Introduction Figure 3 Normal aorta in a male B6C3F1/N mouse from a chronic study. -

Diaphragm Paralysis Caused by Transverse Cervical Artery Compression of the Phrenic Nerve: the Red Cross Syndrome
Clinical Neurology and Neurosurgery 114 (2012) 502–505 Contents lists available at SciVerse ScienceDirect Clinical Neurology and Neurosurgery journal homepage: www.elsevier.com/locate/clineuro Diaphragm paralysis caused by transverse cervical artery compression of the phrenic nerve: The Red Cross syndrome Matthew R. Kaufman a,∗ , Lourens J. Willekes b , Andrew I. Elkwood a , Michael I. Rose a , Tushar R. Patel a , Russell L. Ashinoff a, Alan R. Colicchio c a The Institute for Advanced Reconstruction – 535 Sycamore Avenue, Shrewsbury, NJ 07702, United States b Eastern Thoracic Surgery, 65 Mechanic Street, Red Bank, NJ 07701, United States c Department of Neurology, Jersey Shore University Medical Center – 1944 State Route 33, Neptune, NJ 07753, United States article info abstract Article history: Background: The etiology of diaphragm paralysis is often elusive unless an iatrogenic or traumatic injury Received 16 December 2011 to the phrenic nerve can be clearly implicated. Until recently, there has been little interest in the patho- Received in revised form 17 January 2012 physiology of diaphragm paralysis since few treatment options existed. Accepted 28 January 2012 Methods: We present three cases of symptomatic diaphragm paralysis in which a clear clinico-pathologic Available online 24 February 2012 diagnosis could be identified, specifically a vascular compression of the phrenic nerve in the neck caused by a tortuous or adherent transverse cervical artery. Keywords: Results: In two patients the vascular compression followed a preceding traction injury, whereas in one Phrenic nerve Compression neuropathy patient an inter-scalene nerve block had been performed. Following vascular decompression, all three Diaphragm paralysis patients regained diaphragmatic motion on fluoroscopic chest radiographs, and experienced a resolution Peripheral nerve injury of respiratory symptoms.