Dietary Niche and the Evolution of Cranial Morphology in Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beyond Endocasts: Using Predicted Brain-Structure Volumes of Extinct Birds to Assess Neuroanatomical and Behavioral Inferences
diversity Article Beyond Endocasts: Using Predicted Brain-Structure Volumes of Extinct Birds to Assess Neuroanatomical and Behavioral Inferences 1, , 2 2 Catherine M. Early * y , Ryan C. Ridgely and Lawrence M. Witmer 1 Department of Biological Sciences, Ohio University, Athens, OH 45701, USA 2 Department of Biomedical Sciences, Heritage College of Osteopathic Medicine, Ohio University, Athens, OH 45701, USA; [email protected] (R.C.R.); [email protected] (L.M.W.) * Correspondence: [email protected] Current Address: Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA. y Received: 1 November 2019; Accepted: 30 December 2019; Published: 17 January 2020 Abstract: The shape of the brain influences skull morphology in birds, and both traits are driven by phylogenetic and functional constraints. Studies on avian cranial and neuroanatomical evolution are strengthened by data on extinct birds, but complete, 3D-preserved vertebrate brains are not known from the fossil record, so brain endocasts often serve as proxies. Recent work on extant birds shows that the Wulst and optic lobe faithfully represent the size of their underlying brain structures, both of which are involved in avian visual pathways. The endocasts of seven extinct birds were generated from microCT scans of their skulls to add to an existing sample of endocasts of extant birds, and the surface areas of their Wulsts and optic lobes were measured. A phylogenetic prediction method based on Bayesian inference was used to calculate the volumes of the brain structures of these extinct birds based on the surface areas of their overlying endocast structures. This analysis resulted in hyperpallium volumes of five of these extinct birds and optic tectum volumes of all seven extinct birds. -

P0455-P0459.Pdf
The Condor 101:455-459 0 The Cooper Ornithological Society 1999 BOOK REVIEWS Social Influences on Vocal Development.- Why read the rest of the book if the main themes Charles T Snowdon and Mat-tine Hausberger, eds. are already discussed in the introduction? I believe it 1997. Cambridge University Press, Cambridge, U.K. is worth it, not only to check the statements of the ix + 351 pp. ISBN 0521 49526 1. $95.00 (cloth). editors. All the articles are fine reviews of a given At least in certain aspects, this book is a referee’s field, written by experts. Doug Nelson provides a very dream: not only do the editors provide short summaries good account of the state of the art in song learning in their introduction of the 16 articles making up this theory, and as I expressed above, I fully agree with his volume, they also inform the reader about the main claim that the two stage nature of song learning is still general conclusions that can be drawn from those ar- not fully understood or accepted by other researchers ticles. According to Snowdon and Hausberger, there of the field. are five central themes that arc treated in almost all Luis Baptista and Sandra Gaunt provide a very com- contributions. The first and main theme is the claim petent review on social interaction and vocal devel- that vocal learning has more aspects than learning to opment in birds. As ever, there is a wealth of findings produce sounds. It is also necessary to learn how to from the lab and from the field included in their re- use and comprehend vocalizations. -

Dieter Thomas Tietze Editor How They Arise, Modify and Vanish
Fascinating Life Sciences Dieter Thomas Tietze Editor Bird Species How They Arise, Modify and Vanish Fascinating Life Sciences This interdisciplinary series brings together the most essential and captivating topics in the life sciences. They range from the plant sciences to zoology, from the microbiome to macrobiome, and from basic biology to biotechnology. The series not only highlights fascinating research; it also discusses major challenges associated with the life sciences and related disciplines and outlines future research directions. Individual volumes provide in-depth information, are richly illustrated with photographs, illustrations, and maps, and feature suggestions for further reading or glossaries where appropriate. Interested researchers in all areas of the life sciences, as well as biology enthusiasts, will find the series’ interdisciplinary focus and highly readable volumes especially appealing. More information about this series at http://www.springer.com/series/15408 Dieter Thomas Tietze Editor Bird Species How They Arise, Modify and Vanish Editor Dieter Thomas Tietze Natural History Museum Basel Basel, Switzerland ISSN 2509-6745 ISSN 2509-6753 (electronic) Fascinating Life Sciences ISBN 978-3-319-91688-0 ISBN 978-3-319-91689-7 (eBook) https://doi.org/10.1007/978-3-319-91689-7 Library of Congress Control Number: 2018948152 © The Editor(s) (if applicable) and The Author(s) 2018. This book is an open access publication. Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. -
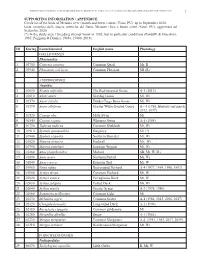
1 ID Euring Latin Binomial English Name Phenology Galliformes
BIRDS OF METAURO RIVER: A GREAT ORNITHOLOGICAL DIVERSITY IN A SMALL ITALIAN URBANIZING BIOTOPE, REQUIRING GREATER PROTECTION 1 SUPPORTING INFORMATION / APPENDICE Check list of the birds of Metauro river (mouth and lower course / Fano, PU), up to September 2020. Lista completa delle specie ornitiche del fiume Metauro (foce e basso corso /Fano, PU), aggiornata ad Settembre 2020. (*) In the study area 1 breeding attempt know in 1985, but in particolar conditions (Pandolfi & Giacchini, 1985; Poggiani & Dionisi, 1988a, 1988b, 2019). ID Euring Latin binomial English name Phenology GALLIFORMES Phasianidae 1 03700 Coturnix coturnix Common Quail Mr, B 2 03940 Phasianus colchicus Common Pheasant SB (R) ANSERIFORMES Anatidae 3 01690 Branta ruficollis The Red-breasted Goose A-1 (2012) 4 01610 Anser anser Greylag Goose Mi, Wi 5 01570 Anser fabalis Tundra/Taiga Bean Goose Mi, Wi 6 01590 Anser albifrons Greater White-fronted Goose A – 4 (1986, february and march 2012, 2017) 7 01520 Cygnus olor Mute Swan Mi 8 01540 Cygnus cygnus Whooper Swan A-1 (1984) 9 01730 Tadorna tadorna Common Shelduck Mr, Wi 10 01910 Spatula querquedula Garganey Mr (*) 11 01940 Spatula clypeata Northern Shoveler Mr, Wi 12 01820 Mareca strepera Gadwall Mr, Wi 13 01790 Mareca penelope Eurasian Wigeon Mr, Wi 14 01860 Anas platyrhynchos Mallard SB, Mr, W (R) 15 01890 Anas acuta Northern Pintail Mi, Wi 16 01840 Anas crecca Eurasian Teal Mr, W 17 01960 Netta rufina Red-crested Pochard A-4 (1977, 1994, 1996, 1997) 18 01980 Aythya ferina Common Pochard Mr, W 19 02020 Aythya nyroca Ferruginous -

Visual Adaptations of Diurnal and Nocturnal Raptors
Seminars in Cell and Developmental Biology 106 (2020) 116–126 Contents lists available at ScienceDirect Seminars in Cell & Developmental Biology journal homepage: www.elsevier.com/locate/semcdb Review Visual adaptations of diurnal and nocturnal raptors T Simon Potiera, Mindaugas Mitkusb, Almut Kelbera,* a Lund Vision Group, Department of Biology, Lund University, Sölvegatan 34, S-22362 Lund, Sweden b Institute of Biosciences, Life Sciences Center, Vilnius University, Saulėtekio Av 7, LT-10257 Vilnius, Lithuania HIGHLIGHTS • Raptors have large eyes allowing for high absolute sensitivity in nocturnal and high acuity in diurnal species. • Diurnal hunters have a deep central and a shallow temporal fovea, scavengers only a central and owls only a temporal fovea. • The spatial resolution of some large raptor species is the highest known among animals, but differs highly among species. • Visual fields of raptors reflect foraging strategies and depend on the divergence of optical axes and on headstructures • More comparative studies on raptor retinae (preferably with non-invasive methods) and on visual pathways are desirable. ARTICLE INFO ABSTRACT Keywords: Raptors have always fascinated mankind, owls for their highly sensitive vision, and eagles for their high visual Pecten acuity. We summarize what is presently known about the eyes as well as the visual abilities of these birds, and Fovea point out knowledge gaps. We discuss visual fields, eye movements, accommodation, ocular media transmit- Resolution tance, spectral sensitivity, retinal anatomy and what is known about visual pathways. The specific adaptations of Sensitivity owls to dim-light vision include large corneal diameters compared to axial (and focal) length, a rod-dominated Visual field retina and low spatial and temporal resolution of vision. -

Bird) Species List
Aves (Bird) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Archosauria, Aves Order (O:) and Family (F:) English Name2 Scientific Name3 O: Tinamiformes (Tinamous) F: Tinamidae (Tinamous) Great Tinamou Tinamus major Highland Tinamou Nothocercus bonapartei O: Galliformes (Turkeys, Pheasants & Quail) F: Cracidae Black Guan Chamaepetes unicolor (Chachalacas, Guans & Curassows) Gray-headed Chachalaca Ortalis cinereiceps F: Odontophoridae (New World Quail) Black-breasted Wood-quail Odontophorus leucolaemus Buffy-crowned Wood-Partridge Dendrortyx leucophrys Marbled Wood-Quail Odontophorus gujanensis Spotted Wood-Quail Odontophorus guttatus O: Suliformes (Cormorants) F: Fregatidae (Frigatebirds) Magnificent Frigatebird Fregata magnificens O: Pelecaniformes (Pelicans, Tropicbirds & Allies) F: Ardeidae (Herons, Egrets & Bitterns) Cattle Egret Bubulcus ibis O: Charadriiformes (Sandpipers & Allies) F: Scolopacidae (Sandpipers) Spotted Sandpiper Actitis macularius O: Gruiformes (Cranes & Allies) F: Rallidae (Rails) Gray-Cowled Wood-Rail Aramides cajaneus O: Accipitriformes (Diurnal Birds of Prey) F: Cathartidae (Vultures & Condors) Black Vulture Coragyps atratus Turkey Vulture Cathartes aura F: Pandionidae (Osprey) Osprey Pandion haliaetus F: Accipitridae (Hawks, Eagles & Kites) Barred Hawk Morphnarchus princeps Broad-winged Hawk Buteo platypterus Double-toothed Kite Harpagus bidentatus Gray-headed Kite Leptodon cayanensis Northern Harrier Circus cyaneus Ornate Hawk-Eagle Spizaetus ornatus Red-tailed -

252 Bird Species
Appendix 5: Fauna Known to Occur on Fort Drum LIST OF FAUNA KNOWN TO OCCUR ON FORT DRUM as of January 2017. Federally listed species are noted with FT (Federal Threatened) and FE (Federal Endangered); state listed species are noted with SSC (Species of Special Concern), ST (State Threatened, and SE (State Endangered); introduced species are noted with I (Introduced). BIRD SPECIES (Taxonomy based on The American Ornithologists’ Union’s 7th Edition Checklist of North American birds.) ORDER ANSERIFORMES ORDER CUCULIFORMES FAMILY ANATIDAE - Ducks & Geese FAMILY CUCULIDAE - Cuckoos Anser albifrons Greater White-fronted Goose Coccyzus americanus Yellow-billed Cuckoo Chen caerulescens Snow Goose Coccyzus erthropthalmusBlack-billed Cuckoo Chen rossii Ross’s Goose Branta bernicla Brant Branta hutchinsii Cackling Goose ORDER CAPRIMULGIFORMES Branta canadensis Canada Goose FAMILY CARPIMULGIDAE - Nightjars Cygnus columbianus Tundra Swan Chordeiles minor Common Nighthawk (SSC) Aix sponsa Wood Duck Antrostomus carolinensis Eastern Whip-poor-will Anas strepera Gadwall (SSC) Anas americana American Wigeon Anas rubripes American Black Duck ORDER APODIFORMES Anas platyrhynchos Mallard FAMILY APODIDAE – Swifts Anas discors Blue-winged Teal Chaetura pelagica Chimney Swift Anas clypeata Northern Shoveler Anas acuta Northern Pintail FAMILY TROCHILIDAE – Hummingbirds Anas crecca Green-winged Teal Archilochus colubris Ruby-throated Hummingbird Aythya valisineria Canvasback Aythya americana Redhead ORDER GRUIFORMES Aythya collaris Ring-necked Duck FAMILY RALLIDAE -

I POPULATION SIZE of BLUE-FOOTED BOOBIES IN
POPULATION SIZE OF BLUE-FOOTED BOOBIES IN GALÁPAGOS: EVALUATION OF INDICATIONS OF POPULATION DECLINE BY DAVID ANCHUNDIA A Thesis Submitted to the Graduate Faculty of WAKE FOREST UNIVERSITY GRADUATE SCHOOL OF ARTS AND SCIENCES in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Biology May 2013 Winston-Salem, North Carolina Approved By: David J. Anderson, Ph.D., Advisor Miles R. Silman, Ph.D., Chair Todd M. Anderson, Ph.D. i DEDICATION This work is dedicated to the memory of my loving mother Juana Isabel Gonzalez. I thank her for all the support and the encouragement she gave me to study sciences. Also I thank my father Oswaldo Anchundia for his constant support during all this time. I will always appreciate all that they have done for me; this degree is dedicated to them. David J. Anchundia Gonzalez ii ACKNOWLEDGEMENT I want to express my sincere gratitude to my advisor Prof. David John Anderson for all the advice, motivation, enthusiasm, and immense knowledge that he shared with me during my M. S. study and research. Also I thank my lab mates Jacquelyn Grace, Felipe Estela, Emily Tompkins, and Terri Mannes for the help and guidance in my research. I want to thank the Prof. Miles Silman and Prof. Michael Anderson, who were part of my thesis committee, and the Professors of the Biology Department from Wake Forest University who shared their knowledge with me. I would like to express my gratitude to: Prof. Kathryn Huyvaert from Colorado State University, who helped me in parts of the analysis and modeling parts of the project; Kyle Anderson from Idaho State University, who helped with part of the GIS analysis; Professors Peter and Rosemary Grant from Princeton University, who provided unpublished breeding and attendance data from Daphne Island; and Lisa Balance and Robert Pitman from the National Marine Fisheries Service (La Jolla) for sharing unpublished at-sea distribution data. -

AOU Classification Committee – North and Middle America
AOU Classification Committee – North and Middle America Proposal Set 2016-C No. Page Title 01 02 Change the English name of Alauda arvensis to Eurasian Skylark 02 06 Recognize Lilian’s Meadowlark Sturnella lilianae as a separate species from S. magna 03 20 Change the English name of Euplectes franciscanus to Northern Red Bishop 04 25 Transfer Sandhill Crane Grus canadensis to Antigone 05 29 Add Rufous-necked Wood-Rail Aramides axillaris to the U.S. list 06 31 Revise our higher-level linear sequence as follows: (a) Move Strigiformes to precede Trogoniformes; (b) Move Accipitriformes to precede Strigiformes; (c) Move Gaviiformes to precede Procellariiformes; (d) Move Eurypygiformes and Phaethontiformes to precede Gaviiformes; (e) Reverse the linear sequence of Podicipediformes and Phoenicopteriformes; (f) Move Pterocliformes and Columbiformes to follow Podicipediformes; (g) Move Cuculiformes, Caprimulgiformes, and Apodiformes to follow Columbiformes; and (h) Move Charadriiformes and Gruiformes to precede Eurypygiformes 07 45 Transfer Neocrex to Mustelirallus 08 48 (a) Split Ardenna from Puffinus, and (b) Revise the linear sequence of species of Ardenna 09 51 Separate Cathartiformes from Accipitriformes 10 58 Recognize Colibri cyanotus as a separate species from C. thalassinus 11 61 Change the English name “Brush-Finch” to “Brushfinch” 12 62 Change the English name of Ramphastos ambiguus 13 63 Split Plain Wren Cantorchilus modestus into three species 14 71 Recognize the genus Cercomacroides (Thamnophilidae) 15 74 Split Oceanodroma cheimomnestes and O. socorroensis from Leach’s Storm- Petrel O. leucorhoa 2016-C-1 N&MA Classification Committee p. 453 Change the English name of Alauda arvensis to Eurasian Skylark There are a dizzying number of larks (Alaudidae) worldwide and a first-time visitor to Africa or Mongolia might confront 10 or more species across several genera. -

Leptosomiformes ~ Trogoniformes ~ Bucerotiformes ~ Piciformes
Birds of the World part 6 Afroaves The core landbirds originating in Africa TELLURAVES: AFROAVES – core landbirds originating in Africa (8 orders) • ORDER ACCIPITRIFORMES – hawks and allies (4 families, 265 species) – Family Cathartidae – New World vultures (7 species) – Family Sagittariidae – secretarybird (1 species) – Family Pandionidae – ospreys (2 species) – Family Accipitridae – kites, hawks, and eagles (255 species) • ORDER STRIGIFORMES – owls (2 families, 241 species) – Family Tytonidae – barn owls (19 species) – Family Strigidae – owls (222 species) • ORDER COLIIFORMES (1 family, 6 species) – Family Coliidae – mousebirds (6 species) • ORDER LEPTOSOMIFORMES (1 family, 1 species) – Family Leptosomidae – cuckoo-roller (1 species) • ORDER TROGONIFORMES (1 family, 43 species) – Family Trogonidae – trogons (43 species) • ORDER BUCEROTIFORMES – hornbills and hoopoes (4 families, 74 species) – Family Upupidae – hoopoes (4 species) – Family Phoeniculidae – wood hoopoes (9 species) – Family Bucorvidae – ground hornbills (2 species) – Family Bucerotidae – hornbills (59 species) • ORDER PICIFORMES – woodpeckers and allies (9 families, 443 species) – Family Galbulidae – jacamars (18 species) – Family Bucconidae – puffbirds (37 species) – Family Capitonidae – New World barbets (15 species) – Family Semnornithidae – toucan barbets (2 species) – Family Ramphastidae – toucans (46 species) – Family Megalaimidae – Asian barbets (32 species) – Family Lybiidae – African barbets (42 species) – Family Indicatoridae – honeyguides (17 species) – Family -

2019 ABA Bird of the Year on the Biology, Field Identification, and General Coolness of the Red-Billed Tropicbird, Phaethon Aethereus
RED-BILLED TROPICBIRD | 2019 BIRD OF THE YEAR 2019 ABA Bird of the Year On the biology, field identification, and general coolness of the Red-billed Tropicbird, Phaethon aethereus IOANA SERITAN Berkeley, California [email protected] PETER PYLE San Francisco, California [email protected] 20 BIRDING | FEBRUARY 2019 he Red-billed Tropicbird is one of of the continental U.S. Read on to learn more Red-billed Tropicbird is joined by the White- three tropicbird species, all of which about tropicbirds in general and Red-billed tailed and Red-tailed tropicbirds to make up Tcan be found in the ABA Area with a Tropicbirds specifically—how to identify the monotypic family Phaethontidae within bit of legwork. Tropicbirds are a fun challenge them, where to find them, and why they are no the order Phaethontiformes. The latest ABA to find, a beauty to look at, and an interesting longer grouped with pelicans. Checklist, updated in December 2018, lists the evolutionary dilemma to consider. You may be Let’s start with some general context on White-tailed Tropicbird as Code 2 (regularly lucky enough to see a pair engaging in court- the tropicbird family. Tropicbirds are pelagic, occurring but range-restricted), while Red- ship display at a breeding site, perhaps meaning “open ocean,” birds that look kind of tailed and Red-billed are both Code 3 (rare in Hawaii, or you may be graced with like glorified terns. Their most famous phys- but annual); before Hawaii was added to the a sighting of a vagrant along ical features are the long tail plumes they ABA Area in late 2016, White-tailed was Code the East or West coast grow as adults. -

South East Brazil, 18Th – 27Th January 2018, by Martin Wootton
South East Brazil 18th – 27th January 2018 Grey-winged Cotinga (AF), Pico da Caledonia – rare, range-restricted, difficult to see, Bird of the Trip Introduction This report covers a short trip to South East Brazil staying at Itororó Eco-lodge managed & owned by Rainer Dungs. Andy Foster of Serra Dos Tucanos guided the small group. Itinerary Thursday 18th January • Nightmare of a travel day with the flight leaving Manchester 30 mins late and then only able to land in Amsterdam at the second attempt due to high winds. Quick sprint (stagger!) across Schiphol airport to get onto the Rio flight which then parked on the tarmac for 2 hours due to the winds. Another roller-coaster ride across a turbulent North Atlantic and we finally arrived in Rio De Janeiro two hours late. Eventually managed to get the free shuttle to the Linx Hotel adjacent to airport Friday 19th January • Collected from the Linx by our very punctual driver (this was to be a theme) and 2.5hour transfer to Itororo Lodge through surprisingly light traffic. Birded the White Trail in the afternoon. Saturday 20th January • All day in Duas Barras & Sumidouro area. Luggage arrived. Sunday 21st January • All day at REGUA (Reserva Ecologica de Guapiacu) – wetlands and surrounding lowland forest. Andy was ill so guided by the very capable REGUA guide Adelei. Short visit late pm to Waldanoor Trail for Frilled Coquette & then return to lodge Monday 22nd January • All day around lodge – Blue Trail (am) & White Trail (pm) Tuesday 23rd January • Early start (& finish) at Pico da Caledonia.