Intertidal and Nearshore Rocky Reefs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INTERTIDAL ZONATION Introduction to Oceanography Spring 2017 The
INTERTIDAL ZONATION Introduction to Oceanography Spring 2017 The Intertidal Zone is the narrow belt along the shoreline lying between the lowest and highest tide marks. The intertidal or littoral zone is subdivided broadly into four vertical zones based on the amount of time the zone is submerged. From highest to lowest, they are Supratidal or Spray Zone Upper Intertidal submergence time Middle Intertidal Littoral Zone influenced by tides Lower Intertidal Subtidal Sublittoral Zone permanently submerged The intertidal zone may also be subdivided on the basis of the vertical distribution of the species that dominate a particular zone. However, zone divisions should, in most cases, be regarded as approximate! No single system of subdivision gives perfectly consistent results everywhere. Please refer to the intertidal zonation scheme given in the attached table (last page). Zonal Distribution of organisms is controlled by PHYSICAL factors (which set the UPPER limit of each zone): 1) tidal range 2) wave exposure or the degree of sheltering from surf 3) type of substrate, e.g., sand, cobble, rock 4) relative time exposed to air (controls overheating, desiccation, and salinity changes). BIOLOGICAL factors (which set the LOWER limit of each zone): 1) predation 2) competition for space 3) adaptation to biological or physical factors of the environment Species dominance patterns change abruptly in response to physical and/or biological factors. For example, tide pools provide permanently submerged areas in higher tidal zones; overhangs provide shaded areas of lower temperature; protected crevices provide permanently moist areas. Such subhabitats within a zone can contain quite different organisms from those typical for the zone. -

Animal Adaptations
Animal Adaptations The Animal Adaptations program at Hatfield Marine Science Center is designed to be a 50- minute lab-based program for 3rd-12th grade students that examines marine organisms from three different habitats (sandy beach, rocky shore and estuary) and explores the many ways they are adapted to their particular environment. This lab focuses on the adaptations of several groups of marine animals including Mollusks, Crustaceans, and Echinoderms, and investigates how they differ depending on whether they are found in a sandy beach, rocky shore, or estuary environment. Students will work in small groups with a variety of live animals, studying individual characteristics and how these organisms interact with their environment and one another. Background Information The Oregon Coast is made up of a series of rocky shores, sandy beaches, and estuaries, all of which are greatly affected by fluctuating tides. Many of these areas are intertidal and are alternately inundated by seawater and exposed to air, wind, and dramatic changes in temperature and salinity. High tide floods these areas with cold, nutrient laden seawater, bringing food to organisms that live there in the form of plankton and detritus. Low tides often expose these organisms to the dangers of predation and desiccation. In addition to tidal effects, organisms that inhabit sandy beaches and rocky shores also have to deal with the physical stresses of pounding waves. Because of these harsh conditions, organisms have developed special adaptations that not only help them to survive but thrive in these environments. An adaptation is a physical or behavioral trait that helps a plant or animal survive in a specific environment or habitat. -

Seashore the SANDYSEASHORE the Soilisinfertile, Anditisoften Windy, Andsalty
SEASHORE DESCRIPTION The seashore is an area fi lled with an interesting mix of unique plants and animals that have adapted to cope with this environment. Living on the edge of the sea is not easy. The soil is infertile, and it is often windy, dry and salty. THE SANDY SEASHORE Along a sandy shore there are no large rocks, algae or tidal pools. The sandy seashore can be divided into four general zones: Intertidal, Pioneer, Fixed Dune and Scrub Woodland. 1. Intertidal Zone: Between the low tide and the high tide mark is the intertidal zone. When the tide goes out the creatures living in this zone are left stranded. They have to endure the heat of the sun and the higher salinity of the water resulting from evaporation. Notice the many small holes on a sandy beach; they are the doorways to the homes of many animals which burrow under the sand where it is cooler. Some of Ecosystems of The Bahamas the creatures living in this zone are sea worms, sand fl eas and sand crabs. 2. Pioneer Zone: So named because it is where the fi rst plants to try to grow over sand. These plants must adapt to loose, shifting sand and poor soil. There is no protection from wind or salt spray. Plants here are usually low growing vines with waxy leaves. Some plants found in the pioneer zone are Purple seaside bean (C. rosea), Saltwort (Batis maritima), Goat's foot (Ipomea pes-caprae), and Sea purslane (Sesuvium portulacastrum). 3. Fixed Dune Zone: The next zone is the fi xed dune, so named because as the plants in the pioneer zone grip the sand around their roots and make the beach more stable, the sand mounds up into small humps or dunes. -

Revised Essd-2020-161 (20 September 2020)
1 1 Half-hourly changes in intertidal temperature at nine wave-exposed 2 locations along the Atlantic Canadian coast: a 5.5-year study 3 Ricardo A. Scrosati, Julius A. Ellrich, Matthew J. Freeman 4 Department of Biology, St. Francis Xavier University, Antigonish, Nova Scotia B2G 2W5, Canada 5 Correspondence to: Ricardo A. Scrosati ([email protected]) 6 Abstract. Intertidal habitats are unique because they spend alternating periods of 7 submergence (at high tide) and emergence (at low tide) every day. Thus, intertidal temperature 8 is mainly driven by sea surface temperature (SST) during high tides and by air temperature 9 during low tides. Because of that, the switch from high to low tides and viceversa can determine 10 rapid changes in intertidal thermal conditions. On cold-temperate shores, which are 11 characterized by cold winters and warm summers, intertidal thermal conditions can also change 12 considerably with seasons. Despite this uniqueness, knowledge on intertidal temperature 13 dynamics is more limited than for open seas. This is especially true for wave-exposed intertidal 14 habitats, which, in addition to the unique properties described above, are also characterized by 15 wave splash being able to moderate intertidal thermal extremes during low tides. To address this 16 knowledge gap, we measured temperature every half hour during a period of 5.5 years (2014- 17 2019) at nine wave-exposed rocky intertidal locations along the Atlantic coast of Nova Scotia, 18 Canada. This data set is freely available from the figshare online repository (Scrosati and 19 Ellrich, 2020a; https://doi.org/10.6084/m9.figshare.12462065.v1). -

PROTISTS Shore and the Waves Are Large, Often the Largest of a Storm Event, and with a Long Period
(seas), and these waves can mobilize boulders. During this phase of the storm the rapid changes in current direction caused by these large, short-period waves generate high accelerative forces, and it is these forces that ultimately can move even large boulders. Traditionally, most rocky-intertidal ecological stud- ies have been conducted on rocky platforms where the substrate is composed of stable basement rock. Projec- tiles tend to be uncommon in these types of habitats, and damage from projectiles is usually light. Perhaps for this reason the role of projectiles in intertidal ecology has received little attention. Boulder-fi eld intertidal zones are as common as, if not more common than, rock plat- forms. In boulder fi elds, projectiles are abundant, and the evidence of damage due to projectiles is obvious. Here projectiles may be one of the most important defi ning physical forces in the habitat. SEE ALSO THE FOLLOWING ARTICLES Geology, Coastal / Habitat Alteration / Hydrodynamic Forces / Wave Exposure FURTHER READING Carstens. T. 1968. Wave forces on boundaries and submerged bodies. Sarsia FIGURE 6 The intertidal zone on the north side of Cape Blanco, 34: 37–60. Oregon. The large, smooth boulders are made of serpentine, while Dayton, P. K. 1971. Competition, disturbance, and community organi- the surrounding rock from which the intertidal platform is formed zation: the provision and subsequent utilization of space in a rocky is sandstone. The smooth boulders are from a source outside the intertidal community. Ecological Monographs 45: 137–159. intertidal zone and were carried into the intertidal zone by waves. Levin, S. A., and R. -

Baja California Sur, Mexico)
Journal of Marine Science and Engineering Article Geomorphology of a Holocene Hurricane Deposit Eroded from Rhyolite Sea Cliffs on Ensenada Almeja (Baja California Sur, Mexico) Markes E. Johnson 1,* , Rigoberto Guardado-France 2, Erlend M. Johnson 3 and Jorge Ledesma-Vázquez 2 1 Geosciences Department, Williams College, Williamstown, MA 01267, USA 2 Facultad de Ciencias Marinas, Universidad Autónoma de Baja California, Ensenada 22800, Baja California, Mexico; [email protected] (R.G.-F.); [email protected] (J.L.-V.) 3 Anthropology Department, Tulane University, New Orleans, LA 70018, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-413-597-2329 Received: 22 May 2019; Accepted: 20 June 2019; Published: 22 June 2019 Abstract: This work advances research on the role of hurricanes in degrading the rocky coastline within Mexico’s Gulf of California, most commonly formed by widespread igneous rocks. Under evaluation is a distinct coastal boulder bed (CBB) derived from banded rhyolite with boulders arrayed in a partial-ring configuration against one side of the headland on Ensenada Almeja (Clam Bay) north of Loreto. Preconditions related to the thickness of rhyolite flows and vertical fissures that intersect the flows at right angles along with the specific gravity of banded rhyolite delimit the size, shape and weight of boulders in the Almeja CBB. Mathematical formulae are applied to calculate the wave height generated by storm surge impacting the headland. The average weight of the 25 largest boulders from a transect nearest the bedrock source amounts to 1200 kg but only 30% of the sample is estimated to exceed a full metric ton in weight. -

Climate Change Report for Gulf of the Farallones and Cordell
Chapter 6 Responses in Marine Habitats Sea Level Rise: Intertidal organisms will respond to sea level rise by shifting their distributions to keep pace with rising sea level. It has been suggested that all but the slowest growing organisms will be able to keep pace with rising sea level (Harley et al. 2006) but few studies have thoroughly examined this phenomenon. As in soft sediment systems, the ability of intertidal organisms to migrate will depend on available upland habitat. If these communities are adjacent to steep coastal bluffs it is unclear if they will be able to colonize this habitat. Further, increased erosion and sedimentation may impede their ability to move. Waves: Greater wave activity (see 3.3.2 Waves) suggests that intertidal and subtidal organisms may experience greater physical forces. A number of studies indicate that the strength of organisms does not always scale with their size (Denny et al. 1985; Carrington 1990; Gaylord et al. 1994; Denny and Kitzes 2005; Gaylord et al. 2008), which can lead to selective removal of larger organisms, influencing size structure and species interactions that depend on size. However, the relationship between offshore significant wave height and hydrodynamic force is not simple. Although local wave height inside the surf zone is a good predictor of wave velocity and force (Gaylord 1999, 2000), the relationship between offshore Hs and intertidal force cannot be expressed via a simple linear relationship (Helmuth and Denny 2003). In many cases (89% of sites examined), elevated offshore wave activity increased force up to a point (Hs > 2-2.5 m), after which force did not increase with wave height. -

Marine Biological Investigations in the Bahamas 23. Description of the Littoral Zonation at Nine Bahamian Rocky-Shore Localities
Marine biological investigations in the Bahamas 23. Description of the littoral zonation at nine Bahamian rocky-shore localities Hans Brattström Brattström H. 1999. Description of the littoral zonation at nine Bahamian rocky-shore localities. SARSIA Sarsia 84:319-365. Nine typical, more or less vertical, rocky-shore localities were visited in April-May 1967, and one of these was revisited in March 1968. The topographies of the stations are described and illustrated, and the vertical zonation of their flora and fauna is documented. On the whole the flora and fauna at the stations investigated was fairly poor. At least 37 species of algae and 85 animal species were recorded, but only 15 algae and 71 animals could be identified to species. Most of the algae were too undeveloped or grazed down to permit a reliable identification. Only half as many species were found at these nine stations as were found on three almost horizontal beach-rock stations described in an earlier article. Whereas many of the latter lived in a system of cavities below the rocks, all species found on the nine vertical stations were exposed to waves and the hot midday sun. The common yellow, black, grey, and white zones typical of the Bahamas, were often well-devel- oped. Some algae and many of the animals formed distinct belts within these zones, the higher up the more exposed the rocks were. Though tides and exposure presumably are responsible for the general zonation pattern, it is likely that in the cirripeds and vermetids the way of food-intake determines their upper limit. -

Life in the Intertidal Zone
Life in the Rocky Intertidal Zone Tidepools provide a home for many animals. Tidepools are created by the changing water level, or tides. The high energy waves make this a harsh habitat, but the animals living here have adapted over time. When the earth, sun and moon align during the full and new moon we have extreme high and low tides. Generally, there are two high tides and two low tides a day. An example of low and high tide is seen on the right. There are three zones within the tidepools: the high zone, the middle zone, and the low zone. Animals are distributed based on their adaptations to different living (competition and predation) and non living (wave action and water loss) factors. The tidepools at Cabrillo are protected and have been monitored by the National Park Service since 1990. You may notice bolts in Low Tide the rocky intertidal, these are used to assist scientists in gathering data to monitor changes. Tidepool Etiquette: Human impact can hurt the animals. As you explore the tidepools, you may touch the animals living here, but only as gently as you would touch your own eyeball. Some animals may die if moved even a few inches from where they are found. Federal law prohibits collection and removal of any shells, rocks and marine specimens. Also, be aware of the changing tides, slippery rocks and unstable cliffs. Have fun exploring! High Tide High Zone The high zone is covered by the highest tides. Often this area is only sprayed by the (Supralittoral or crashing waves. -
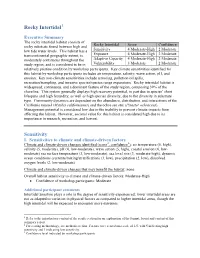
Rocky Intertidal Sensitivity
Rocky Intertidal1 Executive Summary The rocky intertidal habitat consists of Rocky Intertidal Score Confidence rocky substrate found between high and low tide water levels. This habitat has a Sensitivity 4 Moderate-High 2 Moderate transcontinental geographic extent, is Exposure 4 Moderate-High 2 Moderate moderately continuous throughout the Adaptive Capacity 4 Moderate-High 2 Moderate study region, and is considered to be in Vulnerability 3 Moderate 2 Moderate relatively pristine condition by workshop participants. Key climate sensitivities identified for this habitat by workshop participants includes air temperature, salinity, wave action, pH, and erosion. Key non-climate sensitivities include armoring, pollution/oil spills, recreation/trampling, and invasive species/species range expansions. Rocky intertidal habitat is widespread, continuous, and a dominant feature of the study region, composing 39% of the shoreline. This system generally displays high recovery potential, in part due to species’ short lifespans and high fecundity, as well as high species diversity, due to the diversity in substrate type. Community dynamics are dependent on the abundance, distribution, and interactions of the California mussel (Mytilus californianus) and the ochre sea star (Pisaster ochraceus). Management potential is considered low due to the inability to prevent climate impacts from affecting the habitat. However, societal value for this habitat is considered high due to its importance in research, recreation, and harvest. Sensitivity I. Sensitivities -

Spatial Patterns of Macrozoobenthos Assemblages in a Sentinel Coastal Lagoon: Biodiversity and Environmental Drivers
Journal of Marine Science and Engineering Article Spatial Patterns of Macrozoobenthos Assemblages in a Sentinel Coastal Lagoon: Biodiversity and Environmental Drivers Soilam Boutoumit 1,2,* , Oussama Bououarour 1 , Reda El Kamcha 1 , Pierre Pouzet 2 , Bendahhou Zourarah 3, Abdelaziz Benhoussa 1, Mohamed Maanan 2 and Hocein Bazairi 1,4 1 BioBio Research Center, BioEcoGen Laboratory, Faculty of Sciences, Mohammed V University in Rabat, 4 Avenue Ibn Battouta, Rabat 10106, Morocco; [email protected] (O.B.); [email protected] (R.E.K.); [email protected] (A.B.); [email protected] (H.B.) 2 LETG UMR CNRS 6554, University of Nantes, CEDEX 3, 44312 Nantes, France; [email protected] (P.P.); [email protected] (M.M.) 3 Marine Geosciences and Soil Sciences Laboratory, Associated Unit URAC 45, Faculty of Sciences, Chouaib Doukkali University, El Jadida 24000, Morocco; [email protected] 4 Institute of Life and Earth Sciences, Europa Point Campus, University of Gibraltar, Gibraltar GX11 1AA, Gibraltar * Correspondence: [email protected] Abstract: This study presents an assessment of the diversity and spatial distribution of benthic macrofauna communities along the Moulay Bousselham lagoon and discusses the environmental factors contributing to observed patterns. In the autumn of 2018, 68 stations were sampled with three replicates per station in subtidal and intertidal areas. Environmental conditions showed that the range of water temperature was from 25.0 ◦C to 12.3 ◦C, the salinity varied between 38.7 and 3.7, Citation: Boutoumit, S.; Bououarour, while the average of pH values fluctuated between 7.3 and 8.0. -

By the Sea a Guide to the Coastal Zone of Atlantic
BY THE SEA A GUIDE TO THE COASTAL ZONE OF ATLANTIC CANADA USER’S GUIDE ACKNOWLEDGMENTS: FUNDING: Department of Fisheries and Oceans, COORDINATORS: Roland D. Chiasson, Sabine B. Dietz SUPERVISION & REVISIONS: John A. Legault, Sophie Bastien-Daigle GRAPHICS: Sabine Dietz, Ursula Koch, Elke Leitner, Jean-Raymond Gallien (used with permission from the N.B. Department of Natural Resources and Energy) Également disponible en français. Fisheries Pêches Piper Project/Projet siffleur and Oceans et Océans 4800 Route 11 Tabusintac, N.B. E9H 1J6 Department of Fisheries and Oceans Habitat Management Division 343 Archibald Street Moncton, N.B. E1C 9B6 Prepared by: Corvus Consultants Inc., Tabusintac, N.B., Canada Cat. no. S-23-289/1996 E ©1996 ISBN 0-660-16410-8 THE MODULES MODULE 1 : INTRODUCTORY MODULE MODULE 2 : TO THE HORIZON - THE NEARSHORE MODULE 3 : ESTUARIES MODULE 4 : SALT MARSHES MODULE 5 : TIDAL MUDFLATS MODULE 6 : SANDY BEACHES AND DUNES MODULE 7 : ROCKY SHORES MODULE 8 : COASTAL ISLANDS AND CLIFFS MODULE 9 : COBBLE BEACHES MODULE 10 : COASTAL BOGS MODULE 11 : FRESHWATER BARRIER PONDS MODULE 12 : FJORDS MODULE 13 : ACTIVITIES ABOUT THE MANUAL .......................................................................................................................... 3 1.1. Who is this guide for? 3 1.2. How is this guide organized? 3 1.3. About the ecosystem modules 3 1.4. How to use the ecosystem modules: 4 MODULE DESCRIPTIONS, LEARNING OBJECTIVES, STUDY QUESTIONS AND SUGGESTED ACTIVITIES..................... 5 Activity Suggestions 6 Matrix