Study on the Fluoride and Chloride Concentration in Ground Waters of Vilavancode, Kanyakumari District
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Applications for the Post of Night Watchman
List of applications for the post of Night Watchman Receipt Sl. Whether Application is Register Name and address of the applicant No. Accepted (or) Rejected. No. (1) (2) (3) (4) C. Nagarajan, S/o. K.Chellan, 15/15, ElangathuVilai, Palace Road, 1 6373(2) Accepted Thukalay, Kanyakumari District – 629 175. K. Arumugam, S/o. A. Karuppan, 43A/43, Arunthathiyar Street, Age exceeds the maximum 2 6475 Irulappapuram, Kottar Post, Nagercoil. age limit. Hence Rejected. Velayutha Perumal.S., S/o. V. Sudalaiandi, 6C/17, Kulalar Amman Koil Age exceeds the maximum 3 6486 Street, Vagaiadi Street, Kottar, Nagercoil. age limit. Hence Rejected. T. Mahesh, S/o. K. Thalavai, 4/48B, Balavinayagar Koil Street, Thekoor, 4 6497 Accepted Thovalai. D. Jerald, S/o. T. Devaraj, 14-102 L/3, Hookley Garden, Boothapandy Post, 5 6510 Accepted Kanyakumari District-629 852. D.Jebastin, S/o. T.Devaraj, 14-102 L/3, Hookley Garden, Bhoothapandy 6 6518 Accepted Post, Pin 629 852. S. Navaneethan, S/o. S. Saravanan, 3.278C 5/1, Gandhi Colony, 7 6520 Accepted Gurugulam Road, Asaripallam. V. Suresh, S/o. A.Velu, 5/73/4, Ambal Nagar, Kumarapuram, Thoppur, 8 6522 Accepted Kanyakumari District. M. Raju, S/o. S. Murugan, 20A, Manikavasaham Puram, Chettikulam Age exceeds the maximum 9 6532 Junction, Nagercoil. age limit. Hence Rejected A.K. Arul Kumar, 25/1, Pattarkulam Colony, Bhoothapandy Post – 629 852, 10 6589 Accepted Kanyakumari District. S.M. Saravanan, S/o. Sivan Kutty,34/2, 3rd Street, Keezhakulam, 11 6592 Accepted Padmanabhapuram, Thuckalay Post, Kanyakumari District. J. Arun Kumar, S/o. Johnson, Old No.8/41A, New No.8/35, Market Road, 12 6620 Accepted Puliyoorkurichi, Thuckalay Post – 629 175, Kanyakumari District. -

Sl No App.No 1 5291 2 5292 3 5293 4 5294 5 5295 6 5296 7 5297 8 5298
Page 1 of 67 SL APP.NO CANDIDATE NAME NO AND ADDRESS MANIKANDANATH N, S/O NADARAJAN,, PONNARAI, 1 5291 SAHAYA NAGAR, PALAPALLAM (VIA), KANYAKUMARI- 629159 SATHEESH KUMAR K.R, S/O.KUMARADHA S,, 2 5292 VARAGU VILAI,, BETHELPURAM POST,, KANYAKUMARI-0 SHIJU R, S/O.RAMALINGAM NADAR, 3 5293 KAVU VILAI HOUSE,, METHUKUMMAL POST,, S.T.MANKAD, KANYAKUMARI- 629172 ROBINSON R, S/O ROBINSON[L], 4 5294 4-139, APPATTU VILAI, KAPPUKAD POST, KANYAKUMARI- 629162 SELVAKUMAR.T, S/O S.THANGAPPAN, 5 5295 NORTH STREET, MYLAUDY POST, KANYAKUMARI- 629403 NESA RAJA KUMAR, S/O.NESAMON I, 6 5296 KUZHIVILAI HOUSE, THENGAPATTANAM POST, KANYAKUMARI- 0 JAGADEESAN A, S/O AYYAPPA [LATE], 7 5297 1/120B, AKSHARA BAVAN, KRISHNAMANGALAM, THUCKALAY KANYAKUMARI- 629175 MANOHARAN A, S/O ARIKRISHNA PERUMAL, 8 5298 D.NO.3-4,, ATHIKATTU VILAI, MONIKETTIPOTTAL POST- KANYAKUMARI- 629501 Page 2 of 67 NELSON A, S/O ALLECY, 9 5299 KONATHU VILAI, KOODAITHUCKY ROAD, KULASEKHARAM POST KANYAKUMARI- 629161 JOHN BENNET.N, S/O NESAMANI .N 10 5300 MAN PATTAN VILAI, CHERUKOLE, KATTATHURAI POST, KANYAKUMARI- 629158 DAVINSON.C.R, DAVIS COTTAGE, 11 5301 KUTHIRAI VAIKALI VEEDU KOLLAL, KANJAMPURAM POST KANYAKUMARI- 629154 JAYAKUMAR.N, S/O S.NARAYANAN, 12 5302 4-114,PADAR NILAM, VAYAL KARAI, MANAVALAKURICHY POST KANYAKUMARI- 629252 SUNIL T, S/O THANKIAN N 13 5303 KARUMPILA VILAI HOUSE ADAIKKA KUZHI POST KALIYAKKAVILAI VIA KANYAKUMARI- 629153 SASI KUMAR P, S/O PACHAN,, 14 5304 KANCHIRA VILAGAM HOUSE, AYINKAMAMDESOM, KALIAKKAVILAI PO, KANYAKUMARI- 629153 THIYAGARAJAN.T, S/O M.THIRULINGAM, 15 5305 17- 10,CHOTHA VILAI, PUTHALAM POST, KANYAKUMARI- 629602 SREE KUMAR M, S/O.MURUGAN,, 16 5306 POOCHIKATTU VILAI,, THICKANAMCODE POST, KANYAKUMARI-0 Page 3 of 67 MANIGANDAN S, S/O SIVAGURUNATHAN,, 17 5307 19-61B PUVIYOOR,, SOUTH THAMARAIKULAM, AGASTEESWARAM POST. -

Private Schools Fee Determination Committee Chennai-600 006 - Fees Fixed for the Year 2013-2016 - District: Kanyakumari
PRIVATE SCHOOLS FEE DETERMINATION COMMITTEE CHENNAI-600 006 - FEES FIXED FOR THE YEAR 2013-2016 - DISTRICT: KANYAKUMARI SL. SCHOOL HEARING SCHOOL NAME & ADDRESS YEAR LKG UKG I II III IV V VI VII VIII IX X XI XII NO. CODE DATE Artesia Nursery & 2013 - 14 4550 4550 5700 5700 5700 5700 5700 - - - - - - - Primary School, Near St.Antony's 1 010002 Church, 07-05-2013 2014 - 15 5005 5005 6270 6270 6270 6270 6270 - - - - - - - Kappukad Post-629 162, Vilavancod Taluk Kanyakumari D 2015 - 16 5506 5506 6897 6897 6897 6897 6897 - - - - - - - 2013 - 14 3200 3200 3910 3910 3910 3910 3910 - - - - - - - Desiya Vidya Kedra Nursery & Primary 2 010004 School, 07-05-13 2014 - 15 3520 3520 4301 4301 4301 4301 4301 - - - - - - - Villukiri - 629 180. Kanyakumari District. 2015 - 16 3872 3872 4732 4732 4732 4732 4732 - - - - - - - 2013 - 14 4800 4800 6000 6000 6000 6000 6000 - - - - - - - Devi Nursery & Primary School, 3 010005 Kunnamagal, 26-03-13 2014 - 15 5280 5280 6600 6600 6600 6600 6600 - - - - - - - Vaniyakudi P.O Kanyakumari - 629 251 2015 - 16 5808 5808 7260 7260 7260 7260 7260 - - - - - - - 2013 - 14 3200 3200 4200 4200 4200 4200 4200 - - - - - - - Emilin Memorial Nursery & Primary School 4 010006 CSI, Kanjirapuram, 12-03-2013 2014 - 15 3520 3520 4620 4620 4620 4620 4620 - - - - - - - Kanjiracode - 629 155. Kanyakum 2015 - 16 3872 3872 5082 5082 5082 5082 5082 - - - - - - - 2013 - 14 3600 3600 3600 3600 3600 3600 3600 - - - - - - - Good Samaritan Nursery & Primary School, 5 010007 Nediyasalai, 13-03-13 2014 - 15 3960 3960 3960 3960 3960 3960 3960 - - - - - - - Arumanai Post, Kanyakumari District. 2015 - 16 4356 4356 4356 4356 4356 4356 4356 - - - - - - - JOHN PAUL II N & P 2013 - 14 5300 5300 6300 6300 6300 6300 6300 - - - - - - - SCHOOL KULASEKHARAM 6 010009 7-5-13 2014 - 15 5830 5830 6930 6930 6930 6930 6930 - - - - - - - 629 161 ARANIVILAI KANYAKUMARI 2015 - 16 6413 6413 7623 7623 7623 7623 7623 - - - - - - - 1 PRIVATE SCHOOLS FEE DETERMINATION COMMITTEE CHENNAI-600 006 - FEES FIXED FOR THE YEAR 2013-2016 - DISTRICT: KANYAKUMARI SL. -

Rajakkamangalam Thurai Fishing Harbour Pvt., Ltd., Rep
1 BEFORE THE NATIONAL GREEN TRIBUNAL SOUTHERN ZONE, CHENNAI Appeal No.77 of 2015 (SZ) IN THE MATTER OF: Rajakkamangalam Thurai Fishing Harbour Pvt., Ltd., Rep. by its Director, No.26/40, St. Arockia Annai Building, Rajakkamangalam Thurai and Post, Agastheeswaram Taluk, Kanyakumari District. ..Appellant AND 1. Union of India, Rep.by its Secretary, The Ministry of Environment and Forests, New Delhi. 2. The Secretary to Government, Environment and Forest Department, Government of Tamil Nadu, Fort St. George, Chennai-600 009. 3. The Member Secretary/ Director Environment, Tamil Nadu Coastal Zone Management Authority, Panagal Building, Saidapet, Chennai. 2 4. The District Collector, Kanyakumari District, Nagerkoil- 629 001. 5. T. Dharmakrishnan, S/o. Thangiah Nadar, Paniyoor, Ethamozhi Post- 629 501. 6. The Chairman, Paniyoor Region Citizen Welfare Trust, 22-25, Paniyoor, Rajakkamangalam P.O., Kanyakumari District. ...Respondents Counsel appearing for the Appellant : Mr.P.B.Sahasranaman for M/s. TAAURS ASSOCIATES S. Kamaleshkannan and S. Sai Sathya Jith Counsel appearing for the Respondents : Mr. G.M. Syed Nurullah Sheriff for R1 M/s. M.K. Subramanian and P. Velmani for R2 to R4 M/s. Yogeshwaran for Clifton D’ Rozario, Maitreyi Krishnan, Aishwarya Rao & Raghupathy.S for R5 and R6 3 J U D G E M E NT PRESENT: HON’BLE SHRI JUSTICE M.S.NAMBIAR, JUDICIAL MEMBER HON’BLE SHRI P.S. RAO, EXPERT MEMBER Delivered by Hon’ble Justice M.S.NAMBIAR , Judicial Member Dated:23rd December, 2016 ------------------------------------------------------------------------------- Whether the Judgement is allowed to be published on the Internet – Yes/No Whether the Judgement is to be published in the All India NGT Reporter – Yes/No The Rajakkamangalam Thurai Development Trust by its representation dated 30.04.2004 requested the Government of Tamil Nadu to construct a Fishing Harbour at Rajakkamangalam Thurai in Kanyakumari District under BOOT (Build-Own-Operate- Transfer) system. -

BEFORE the NATIONAL GREEN TRIBUNAL SOUTHERN ZONE, CHENNAI. REVIEW APPLICATION No
BEFORE THE NATIONAL GREEN TRIBUNAL SOUTHERN ZONE, CHENNAI. REVIEW APPLICATION No: 22 OF 2015(SZ) IN APPLICATION No.05 OF 2013 (SZ) IN THE MATTER OF: M/s. Pannaiyoor Regional Citizens Welfare Trust Rep. by its Secretary 22-25, Pannaiyoor Rajakkamangalam P.O Kanyakumari District. ... Applicants Versus 1. The Ministry of Environment, Forests and Climate Change Union of India Rep. by its Secretary CGO Complex, Lodhi Road New Delhi-110 003. 2. Tamil Nadu Pollution Control Board Rep. by its Chairman Anna Salai Chennai-600 032. 3. The District Environmental Engineer Tamil Nadu Pollution Control Board No.30, Kesari Street Mathias Nagar Nagercoil. 4. The District Collector Kanyakumari District District Collectorate Complex Kanyakumari. 5. M/s. Rajakkamangalam Thurai Fishing Harbour Pvt. Ltd. Rajakkamangalam Thurai Kanyakumari District Tamil Nadu 6. The Tamil Nadu State Coastal Zone Management Authority Rep. by its Member Secretary Panagal Building, Saidapet Chennai. 7. The Principal Chief Conservator of Forest Head of Forest Force Panagal Building Saidapet Chennai. .... Respondents REVIEW APPLICATION No: 23 OF 2015(SZ) IN APPLICATION No.04 OF 2014 (SZ) IN THE MATTER OF: Mr. Dharmakrishnan Son of Thangiah Nadar Pannaiyur Ethamozhi Post-629 501 Kanyakumari District ... Applicant Versus 1. The Principal Chief Conservator of Forest Head of Forest Force Panagal Building Saidapet Chennai. 2. The Secretary to Government Environment and Forest Department Government of Tamil Nadu Fort St. George Chennai.600 009. 3. The Secretary to Government Animal Husbandry Dairying and Fisheries (FSI) Department Government of Tamil Nadu Fort St. George Chennai-600 009. 4. The Commissioner of Fisheries Government of Tamil Nadu DMS Complex, Teynampet Chennai-600 006 5. -

Kodaiyar River Basin
Kodaiyar River Basin Introduction One of the oldest systems in Tamil Nadu is the “Kodaiyar system” providing irrigation facilities for two paddy crop seasons in Kanyakumari district. The Kodaiyar system comprises the integrated operation of commands of two major rivers namely Pazhayar and Paralayar along with Tambaraparani or Kuzhithuraiyur in which Kodaiyar is a major tributary. The whole system is called as Kodaiyar system. Planning, development and management of natural resources in this basin require time-effective and authentic data.The water demand for domestic, irrigation, industries, livestock, power generation and public purpose is governed by socio – economic and cultural factors such as present and future population size, income level, urbanization, markets, prices, cropping patterns etc. Water Resources Planning is people oriented and resources based. Data relating to geology, geomorphology, hydrogeology, hydrology, climatology, water quality, environment, socio – economic, agricultural, population, livestock, industries, etc. are collected for analysis. For the sake of consistency, other types of data should be treated in the same way. Socio – economic, agricultural and livestock statistics are collected and presented on the basis of administrative units located within this basin area. Location and extent of Kodaiyar Basin The Kodaiyar river basin forms the southernmost end of Indian peninsula. The basin covers an area of 1646.964 sq km. The flanks of the entire basin falls within the TamilnaduState boundary. Tamiraparani basin lies on the north and Kodaiyar basin on the east and Neyyar basin of Kerala State lies on the west. This is the only river basin which has its coastal border adjoining the Arabian sea, the Indian Ocean in the south and the Gulf of Mannar in the east. -

Lithological Studies and Types of Soils Along Coastal Area of Kanyakumari to Colachel Coastal Belt, South Tamil Nadu, India
Nature Environment and Pollution Technology Vol. 7 No. 4 pp. 00-00 2008 An International Quarterly Scientific Journal Original Research Paper Lithological Studies and Types of Soils Along Coastal Area of Kanyakumari to Colachel Coastal Belt, South Tamil Nadu, India S. Bhagavathi Perumal and P. Thamarai* Department of Civil Engineering, National College of Engineering, Tirunelveli-627 151, T.N. *Department of Civil Engineering, Government College of Engineering, Tirunelveli-627 007, T.N. ABSTRACT Key Words: Lithological studies Soil types of an area are more important, since soil is the main criteria in agricultural Coastal area production and in recharge of groundwater. Different types of soils are derived Kanyakumari from a wide range of geological formations. Knowledge about type of soils, its Colachel coastal belt extent and occurrence is of primary importance for agricultural planning to maximize production and for groundwater recharge. The soils of the district from Kanyakumari to Colachel coastal belt can be classified into red loams, red lateritic soil and pale reddish. They are derived from lateralization of gneisses. The soils derived from gneisses are mostly brownish. Thickness of soils in mountains is almost negligible, whereas in the valleys they are around 2m thick. The lateritic type of soil occurs in blocks of Rajakkamangalam. The mixed type of red and alluvial soil occurs in Agastheeswaram block. The coastal sand occurs in the western side of the district. The coastal alluvium sand is of high fertility. Generally, the soils are highly acidic in the coastal area. This is mainly due to heavy rainfall and heavy leaching of basic rock in hilly areas. -

Communication Address Name of Enterprise 1 THAMPI
Communication Address Name of Enterprise UTHRAM, LEKSHMI VLAKAM, 1 THAMPI POWERLOOM BHAGAVATHY NADAYIL NILAMEL NALUKETTU TC 6/525/1 2 MITRA NAGAR VATTIYOORKAVU PO SAFA 695013 TC 11/2750 PANANVILA NALANCHIRA NANDANA BAKERS & FRESH 3 PO TVPM 695015 JUICE ELAVUNKAL STEP JUNCTION 4 MADONNA NALANCHIRA PO TV[,695015 TC54/2331 PADMANABHA 5 ADRIKA INFO SOLUTIONS PAPPANAMCODE PO TVPM 695018 SIJI MANZIL THONNAKKAL PO 6 WESTERN PUTTUPODI MANGALAPURAM GANAM TC 5/2067/14 VGRA-4 7 GLACE JEWELLERY DESIGNS KOWDIAR PO TVPM-695003 CHALISA NRRA-118/1 NETAJI ROAD 8 RESHAM GARMENTS VATTIYOORKAVU KP VIII/292 ALAMCODE 9 SHA ENGINEERING WORKS CHIRAYINKEEZHU TVPM-695102 TC15/1158 GANDHI NAGAR 10 9th SENSE MEDIA HUB THYCAUD TVPM HOUSE NO.137 NEAR WATER TANK 11 EKTON ENGINEERING WORKS PALLITHURA PO TVPM-695586 SREE AYILYAM SNRA-106 SOORYA 12 NAGAR KALAKAUMUDHI RD. VKS EXPORTERS KUMARAPURAM-695011 PANAMOOTTIL VEEDU 13 KOTTARAKONAM VENJARAMOODU PO DEVI ENGINEERING WORKS 695607 OXY SMART SERVICE VALICODE NDD- 14 KERALA GRAPHICS 695541 LATHA BHAVAN ALAMCODE ANAD PO PRIYA SOUNDS AND 15 NDD ELECTRICAL WORKS SAGARA THRIPPADAPURAM NORTH 16 MAGIK STROKZ KULATHOOR PO TVPM-695583 KUZHIVILA VEEDU CHEMMARUTHY 17 CHIKKU INDUSTRIES VADASSERIKONAM P O-695143 NEELANJANAM VPIX/511 C-SEC(CENTRE FOR SOCIAL 18 PANAAMKARA KODUNGANOOR P O AND ECOLOGICAL CARE) VATTIYOORKAVU-695013 ZENITH COTTAGE CHATHANPARA GURUPRASADAM READYMADE 19 THOTTAKKADU PO PIN695605 GARMENTS KARTHIKA VP 9/669 20 KODUNGANOORPO KULASEKHARAM GEETHAM 695013 SHAMLA MANZIL ARUKIL, 21 KUNNUMPURAM KUTTICHAL PO- N A R FLOUR MILLS 695574 RENVIL APARTMENTS TC1/1517 22 NAVARANGAM LANE MEDICAL VIJU ENTERPRISE COLLEGE PO NIKUNJAM, KRA-94,KEDARAM CORGENTZ INFOTECH PRIVATE 23 NAGAR,PATTOM PO, TRIVANDRUM LIMITED KALLUVELIL HOUSE KANDAMTHITTA 24 AMALA AYURVEDIC PHARMA PANTHA PO TVM PUTHEN PURACKAL KP IV/450-C 25 NEAR AL-UTHMAN SCHOOL AARC METAL AND WOOD MENAMKULAM TVPM KINAVU HOUSE TC 18/913 (4) 26 KALYANI DRESS WORLD ARAMADA PO TVPM THAZHE VILAYIL VEEDU OPP. -

Kanniyakumari (D) - Vilavancode (T) Center Details
Tamilnadu e-Governance Agency No. 5/9, TNHB Building,Kavingar Bharathidasan Road, Cresent Street, Alwarpet, Chennai - 600 018. Kanniyakumari (D) - Vilavancode (T) Center Details S. Taluk Firka Center Name Agency Village Name Address No Name Name 1 Vayakkalloor - PACS PACS Vilavancode Painkulam Ezhudesam C 18/174F, Near to SBI, Vayakkalloor, Kanjampuram, Ezhudesam - 629154 2 Kollemcode - PACS PACS Vilavancode Painkulam Kollancode A Opp. to Panchayat Office, Kollemcode, Siluvaipuram - 629160 3 Kurumathoor - PACS PACS Vilavancode Vilavancode Kaliyakkavilai 23/80, Thiruthuvapuram, Kuzhithurai, Kurumathoor - 629163 4 Elanchirai - PACS PACS Vilavancode Edaicode Edaicode 27/72-A, Near to Post Office, Elanchirai - 629152 5 Arumanai - PACS PACS Vilavancode Arumanai Vellamcode 14824, Cooperative Bank Stop, Arumanai - 629151 6 Palugal - PACS PACS Vilavancode Edaicode Palugal 12/143, Opp. to VAO Office, Karumanoor, Palugal - 629170 7 Anducode - PACS PACS Vilavancode Edaicode Anducode A 22/26, Opp. to VAO Office, Anducode - 629168 8 Vilavancode - PACS PACS Vilavancode Vilavancode Kuzhithurai Town 12-12/A5, Kalluketti Junction, Kalluketti, Kuzhithurai - 629163 9 Munchirai PCARD Bank - PACS PACS Vilavancode Vilavancode Kuzhithurai Town 203/12A, Post Office Junction, Palace Ward, Kuzhithurai - 629163 10 Kuzhithurai HCS - PACS PACS Vilavancode Vilavancode Kuzhithurai Town 15/83C, Taluk office Road, Kuzhithurai - 629163 11 Pacode - PACS PACS Vilavancode Edaicode Maruthancode Near Melpuram Junction, Melpuram - 629168 12 Parthipapuram Munchirai - PACS PACS Vilavancode Painkulam Munchirai 3/116, Opp. to J.R.Mahal, Puthukadai, Ottapilavilai - 629171 13 Kirathoor - PACS PACS Vilavancode Vilavancode Ezhudesam C Poonthottam, Nithiravilai, Kirathoor - 629154 14 Puthukadai HCS - PACS PACS Vilavancode Painkulam Munchirai 9/106, Near to Auto Stand, Kazhunettanvilai, Puthukadai - 629171 15 Vencode - PACS PACS Vilavancode Vilavancode Killiyoor B Near to R.C. -

Kanyakumari District, Tamil Nadu
For official use Technical Report Series DISTRICT GROUNDWATER BROCHURE KANYAKUMARI DISTRICT, TAMIL NADU A. Balachandran Scientist - D Government of India Ministry of Water Resources Central Ground Water Board South Eastern Coastal Region Chennai September 2008 0 DISTRICT AT A GLANCE (KANYAKUMARI) S. No. ITEMS STATISTICS 1. GENERAL INFORMATION i. Geographical area (Sq. km) 1671.84 ii. Administrative Divisions (As on 31-3-2007) Number of Taluks 4 Number of Blocks 9 Number of Villages 81 iii. Population (As on 2001 Censes) Total Population 1676034 Male 832269 Female 843765 iv. Average Annual Rainfall (mm) 1448.6 2. GEOMORPHOLOGY i. Major physiographic Units (i). Western Ghats (ii). Coastal Plain. ii. Major Drainages Pazhayar, Valliyar & Tamirabarani. 3. LAND USE (Sq. km) (2005-06) i. Forest area 541.55 ii. Net area sown 793.23 iii. Barren & Uncultivable waste 31.49 4. MAJOR SOIL TYPES 1. Red soil 2. Lateritic soil 3. Clayey soil, 4. River Alluvium & 5. Coastal Alluvium. 5. AREA UNDER PRINCIPAL CROPS (Ha.) 1. Paddy -21709 (56%) (2005-2006) 1. Coconut – 9388 (24%) 2. Banana – 5509 (14.2%) 4. Pulses – 166 (< 1 %) 6. IRRIGATION BY DIFFERENT SOURCES (2005-06) Number Area irrigated (Ha.) i. Dug wells 3349 1535 ii. Tube wells 1303 913 iii. Tanks 2623 13657 iv. Canals 53 22542 vi. Net irrigated area 27694 Ha. vii. Gross irrigated area 38885 Ha. 1 7. NUMBER OF GROUND WATER MONITORING WELLS OF CGWB (As on 31.03.2007) i. Dug wells 14 ii. Piezometers 8 8. PREDOMINANT GEOLOGICAL Recent Alluvium, & Warkalai FORMATIONS Sandstones, Peninsular Gneisses Charnockites, Khondalites, Granites and Pegmatites 9. -
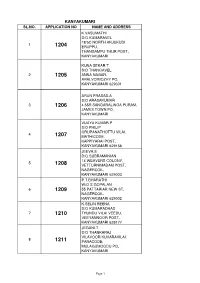
Kanyakumari Sl.No
KANYAKUMARI SL.NO. APPLICATION NO NAME AND ADDRESS K.VASUMATHI D/O KUMARAVEL 18/5C,NORTH ANJUKUDI 1 1204 ERUPPU, THANGAMPU THUR POST, KANYAKUMARI KUNA SEKAR.T S/O THANGAVEL 2 1205 ANNA NAGAR, ARALVOIMOZHY PO, KANYAKUMARI 629301 ARUN PRASAD.A S/O ARASAKUMAR 3 1206 4.55R SANGARALINGA PURAM, JAMES TOWN PO, KANYAKUMARI VIJAYA KUMAR.P S/O PHILIP ORUPANATHOTTU VILAI, 4 1207 MATHICODE, KAPPIYARAI POST, KANYAKUMARI 629156 JEEVA.S D/O SUBRAMANIAN 14 WEAVERS COLONY, 5 1208 VETTURNIMADAM POST, NAGERCOIL, KANYAKUMARI 629003 P.T.BHARATHI W/O S.GOPALAN 6 1209 55 PATTARIAR NEW ST, NAGERCOIL, KANYAKUMARI 629002 K.SELIN REENA D/O KUMARADHAS 7 1210 THUNDU VILAI VEEDU, VEEYANNOOR POST, KANYAKUMARI 629177 JEGANI.T D/O THANKARAJ VILAVOOR KUVARAVILAI, 8 1211 PARACODE, MULAGUMOODU PO, KANYAKUMARI Page 1 FELSY FREEDA.L D/O LAZER 9 1212 MALAANTHATTU VILAI, PALLIYADI POST, KANYAKUMARI 629169 CHRISTAL KAVITHA. R D/O RAJAN 10 1213 VALIYAVILAGAM HOUSE ST, MANKADU POST, KANYAKUMARI 629172 PRABHA.P D/O PADMANABHAN 11 1214 EATHENKADU, FRIDAYMARKET POST, KANYAKUMARI 629802 KALAI SELVI.N D/O NARAYANA PERUMAL 12 1215 THERIVILAI SWAMITHOPPU POST, KANYAKUMARI 629704 NAGALAKSHMI.M D/O MURUGESAN LEKSHMI BHAVAN, 13 1216 CHAKKIYANCODE, NEYYOOR POST, KANYAKUMARI 629802 BEULA.S D/O SATHIADHAS 14 1217 ANAN VILAI, KEEZHKULAM PO, KANYAKUMARI 629193 MAHESWARI.S D/O SIVACHANDRESWARAN 15 1218 1/20BTHEKKURICHI, RAJAKKAMANGALAM POST, KANYAKUMARI 629503 PREMALATHA.S W/O MURALIRAJ V.L, 460F-1, M.S.ROAD, 16 1219 SINGARATHOPPUPAR, VATHIPURAM, NAGERCOIL, KANYAKUMARI 629003 SUBASH.T S/O THANKAPPAN MANALI KATTU VILAI, 17 1220 PUTHEN VEEDU THICKA, NAMCODE PO, KANYAKUMARI 629804 Page 2 J. -

List of Town Panchayats Name in Tamil Nadu Page 1 District Code
List of Town Panchayats Name in Tamil Nadu Sl. No. District Code District Name Town Panchayat Name 1 1 KANCHEEPURAM ACHARAPAKKAM 2 1 KANCHEEPURAM CHITLAPAKKAM 3 1 KANCHEEPURAM EDAKALINADU 4 1 KANCHEEPURAM KARUNGUZHI 5 1 KANCHEEPURAM KUNDRATHUR 6 1 KANCHEEPURAM MADAMBAKKAM 7 1 KANCHEEPURAM MAMALLAPURAM 8 1 KANCHEEPURAM MANGADU 9 1 KANCHEEPURAM MEENAMBAKKAM 10 1 KANCHEEPURAM NANDAMBAKKAM 11 1 KANCHEEPURAM NANDIVARAM - GUDUVANCHERI 12 1 KANCHEEPURAM PALLIKARANAI 13 1 KANCHEEPURAM PEERKANKARANAI 14 1 KANCHEEPURAM PERUNGALATHUR 15 1 KANCHEEPURAM PERUNGUDI 16 1 KANCHEEPURAM SEMBAKKAM 17 1 KANCHEEPURAM SEVILIMEDU 18 1 KANCHEEPURAM SHOLINGANALLUR 19 1 KANCHEEPURAM SRIPERUMBUDUR 20 1 KANCHEEPURAM THIRUNEERMALAI 21 1 KANCHEEPURAM THIRUPORUR 22 1 KANCHEEPURAM TIRUKALUKUNDRAM 23 1 KANCHEEPURAM UTHIRAMERUR 24 1 KANCHEEPURAM WALAJABAD 25 2 TIRUVALLUR ARANI 26 2 TIRUVALLUR CHINNASEKKADU 27 2 TIRUVALLUR GUMMIDIPOONDI 28 2 TIRUVALLUR MINJUR 29 2 TIRUVALLUR NARAVARIKUPPAM 30 2 TIRUVALLUR PALLIPATTU 31 2 TIRUVALLUR PONNERI 32 2 TIRUVALLUR PORUR 33 2 TIRUVALLUR POTHATTURPETTAI 34 2 TIRUVALLUR PUZHAL 35 2 TIRUVALLUR THIRUMAZHISAI 36 2 TIRUVALLUR THIRUNINDRAVUR 37 2 TIRUVALLUR UTHUKKOTTAI Page 1 List of Town Panchayats Name in Tamil Nadu Sl. No. District Code District Name Town Panchayat Name 38 3 CUDDALORE ANNAMALAI NAGAR 39 3 CUDDALORE BHUVANAGIRI 40 3 CUDDALORE GANGAIKONDAN 41 3 CUDDALORE KATTUMANNARKOIL 42 3 CUDDALORE KILLAI 43 3 CUDDALORE KURINJIPADI 44 3 CUDDALORE LALPET 45 3 CUDDALORE MANGALAMPET 46 3 CUDDALORE MELPATTAMPAKKAM 47 3 CUDDALORE PARANGIPETTAI