Quelea Birds in Southern Africa: Protocols for Environmental Assessment of Control and Models for Breeding Forecasts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species List (Note, There Was a Pre-Tour to Kenya in 2018 As in 2017, but These Species Were Not Recorded
Tanzania Species List (Note, there was a pre-tour to Kenya in 2018 as in 2017, but these species were not recorded. You can find a Kenya list with the fully annotated 2017 Species List for reference) February 6-18, 2018 Guides: Preston Mutinda and Peg Abbott, Driver/guides William Laiser and John Shoo, and 6 participants: Rob & Anita, Susan and Jan, and Bob and Joan KEYS FOR THIS LIST The # in (#) is the number of days the species was seen on the tour (E) – endemic BIRDS STRUTHIONIDAE: OSTRICHES OSTRICH Struthio camelus massaicus – (8) ANATIDAE: DUCKS & GEESE WHITE-FACED WHISTLING-DUCK Dendrocygna viduata – (2) FULVOUS WHISTLING-DUCK Dendrocygna bicolor – (1) COMB DUCK Sarkidiornis melanotos – (1) EGYPTIAN GOOSE Alopochen aegyptiaca – (12) SPUR-WINGED GOOSE Plectropterus gambensis – (2) RED-BILLED DUCK Anas erythrorhyncha – (4) HOTTENTOT TEAL Anas hottentota – (2) CAPE TEAL Anas capensis – (2) NUMIDIDAE: GUINEAFOWL HELMETED GUINEAFOWL Numida meleagris – (12) PHASIANIDAE: PHEASANTS, GROUSE, AND ALLIES COQUI FRANCOLIN Francolinus coqui – (2) CRESTED FRANCOLIN Francolinus sephaena – (2) HILDEBRANDT'S FRANCOLIN Francolinus hildebrandti – (3) Naturalist Journeys [email protected] 866.900.1146 / Caligo Ventures [email protected] 800.426.7781 naturalistjourneys.com / caligo.com P.O. Box 16545 Portal AZ 85632 FAX: 650.471.7667 YELLOW-NECKED FRANCOLIN Francolinus leucoscepus – (4) [E] GRAY-BREASTED FRANCOLIN Francolinus rufopictus – (4) RED-NECKED FRANCOLIN Francolinus afer – (2) LITTLE GREBE Tachybaptus ruficollis – (1) PHOENICOPTERIDAE:FLAMINGOS -

Species Limits in the Indigobirds (Ploceidae, Vidua) of West Africa: Mouth Mimicry, Song Mimicry, and Description of New Species
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 162 Species Limits in the Indigobirds (Ploceidae, Vidua) of West Africa: Mouth Mimicry, Song Mimicry, and Description of New Species Robert B. Payne Museum of Zoology The University of Michigan Ann Arbor, Michigan 48109 Ann Arbor MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN May 26, 1982 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN The publications of the Museum of Zoology, University of Michigan, consist of two series-the Occasional Papers and the Miscellaneous Publications. Both series were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W. W. Newcomb. The Occasional Papers, publication of which was begun in 1913, serve as a medium for original studies based principally upon the collections in the Museum. They are issued separately. When a sufficient number of pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and individuals on the mailing list for the series. The Miscellaneous Publications, which include papers on field and museum techniques, monographic studies, and other contributions not within the scope of the Occasional Papers, are published separately. It is not intended that they be grouped into volumes. Each number has a title page and, when necessary, a table of contents. A complete list of publications on Birds, Fishes, Insects, Mammals, Mollusks, and Reptiles and Amphibians is available. Address inquiries to the Director, Museum of Zoology, Ann Arbor, Michigan 48109. MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 162 Species Limits in the Indigobirds (Ploceidae, Vidua) of West Africa: Mouth Mimicry, Song Mimicry, and Description of New Species Robert B. -

Download Download
Biodiversity Observations http://bo.adu.org.za An electronic journal published by the Animal Demography Unit at the University of Cape Town The scope of Biodiversity Observations consists of papers describing observations about biodiversity in general, including animals, plants, algae and fungi. This includes observations of behaviour, breeding and flowering patterns, distributions and range extensions, foraging, food, movement, measurements, habitat and colouration/plumage variations. Biotic interactions such as pollination, fruit dispersal, herbivory and predation fall within the scope, as well as the use of indigenous and exotic species by humans. Observations of naturalised plants and animals will also be considered. Biodiversity Observations will also publish a variety of other interesting or relevant biodiversity material: reports of projects and conferences, annotated checklists for a site or region, specialist bibliographies, book reviews and any other appropriate material. Further details and guidelines to authors are on this website. Paper Editor: Les G. Underhill OVERVIEW OF THE DISCOVERY OF THE WEAVERS H. Dieter Oschadleus Recommended citation format: Oschadleus HD 2016. Overview of the discovery of the weavers. Biodiversity Observations 7. 92: 1–15. URL: http://bo.adu.org.za/content.php?id=285 Published online: 13 December 2016 – ISSN 2219-0341 – Biodiversity Observations 7.92: 1–15 1 TAXONOMY Currently, 117 living species of weavers in the Ploceidae family are recognised. Hoyo et al. OVERVIEW OF THE DISCOVERY OF THE WEAVERS (2010) listed 116 species but Safford & Hawkins (2013) split the Aldabra Fody Foudia H. Dieter Oschadleus aldabrana from the Red- headed Fody Foudia Animal Demography Unit, Department of Biological Sciences, eminentissima. Dickinson & University of Cape Town, Rondebosch, 7701 South Africa Christidis (2014) also listed 117 species. -

Bontebok Birds
Birds recorded in the Bontebok National Park 8 Little Grebe 446 European Roller 55 White-breasted Cormorant 451 African Hoopoe 58 Reed Cormorant 465 Acacia Pied Barbet 60 African Darter 469 Red-fronted Tinkerbird * 62 Grey Heron 474 Greater Honeyguide 63 Black-headed Heron 476 Lesser Honeyguide 65 Purple Heron 480 Ground Woodpecker 66 Great Egret 486 Cardinal Woodpecker 68 Yellow-billed Egret 488 Olive Woodpecker 71 Cattle Egret 494 Rufous-naped Lark * 81 Hamerkop 495 Cape Clapper Lark 83 White Stork n/a Agulhas Longbilled Lark 84 Black Stork 502 Karoo Lark 91 African Sacred Ibis 504 Red Lark * 94 Hadeda Ibis 506 Spike-heeled Lark 95 African Spoonbill 507 Red-capped Lark 102 Egyptian Goose 512 Thick-billed Lark 103 South African Shelduck 518 Barn Swallow 104 Yellow-billed Duck 520 White-throated Swallow 105 African Black Duck 523 Pearl-breasted Swallow 106 Cape Teal 526 Greater Striped Swallow 108 Red-billed Teal 529 Rock Martin 112 Cape Shoveler 530 Common House-Martin 113 Southern Pochard 533 Brown-throated Martin 116 Spur-winged Goose 534 Banded Martin 118 Secretarybird 536 Black Sawwing 122 Cape Vulture 541 Fork-tailed Drongo 126 Black (Yellow-billed) Kite 547 Cape Crow 127 Black-shouldered Kite 548 Pied Crow 131 Verreauxs' Eagle 550 White-necked Raven 136 Booted Eagle 551 Grey Tit 140 Martial Eagle 557 Cape Penduline-Tit 148 African Fish-Eagle 566 Cape Bulbul 149 Steppe Buzzard 572 Sombre Greenbul 152 Jackal Buzzard 577 Olive Thrush 155 Rufous-chested Sparrowhawk 582 Sentinel Rock-Thrush 158 Black Sparrowhawk 587 Capped Wheatear -

Download Report
Tel office: +264 61 379 513 Physical address: Postal address: Fax office: +264 61 22 5371 Agricultural Boards’ Building PO Box 5096 E-mail: [email protected] 30 David Hosea Meroro Road Ausspannplatz Website: www.nab.com.na Windhoek Windhoek Constituted by Act 20 of 1992 Creating a marketing environment that is conducive to growing and processing crops in Namibia AGRONOMY AND HORTICULTURE MARKET DEVELOPMENT DIVISION RESEARCH AND POLICY DEVELOPMENT SUBDIVISION QUELEA BIRDS CONTROL MEASURES IN NAMIBIA: TRIAL USING AGRI-FREQUENCY METHOD 2019 Quelea quelea birds control - Research Report 2019 Page 0 of 11 TABLE OF CONTENTS 1. BACKGROUND ............................................................................................................ 2 2. OBJECTIVES ................................................................................................................. 2 3. METHODOLOGY ......................................................................................................... 2 4. DESCRIPTION............................................................................................................... 2 5. BREEDING .................................................................................................................... 3 6. HABITAT ....................................................................................................................... 4 7. FEEDING ....................................................................................................................... 4 8. POPULATION .............................................................................................................. -

Bird List Please Inform Us of Any Confirmed Sightings of Birds Not Listed Here to Help Us Expand Our List!
Bird List Please inform us of any confirmed sightings of birds not listed here to help us expand our list! Apalis, Bar-throated Crombec, Long-billed Goshawk, Dark Chanting Apalis, Yellow-breasted Crow, Cape Grassbird, Cape Babbler, Arrow-marked Crow, Pied Grebe, Little Barbet, Acacia Pied Cuckoo, African Greenbul, Sombre Barbet, Black-collared Cuckoo, Black Greenbul, Yellow-bellied Barbet, Crested Cuckoo, Diederick Guineafowl, Helmeted Batis, Cape Cuckoo, Jacobin Hamerkop Batis, Chinspot Cuckoo, Klaas’s Harrier, Montagu’s Bee-eater, European Cuckoo, Levaillant’s Hawk, African Harrier- Bee-eater, Little Cuckoo, Red-chested Hawk, Bat Bee-eater, White-fronted Cuckoo, Great Spotted Helmet-shrike, Retz’s Bishop, Southern Red Cuckooshrike, Black Helmet-Shrike, White- Bishop, Yellow Darter, African crested Boubou, Southern Dove, Laughing Heron, Black-headed Brownbul, Terrestrial Dove, Lemon Heron, Grey Brubru Dove, Namaqua Heron, Goliath Bulbul, Dark-capped Dove, Red-eyed Heron, Green-backed Bunting, Cinnamon-breasted Dove, Ring-necked (Cape Heron, Purple Bunting, Golden-breasted Turtle) Hobby, Eurasian Bush-Shrike, Gorgeous Dove, Tambourine Honeybird, Brown- Bush-Shrike, Grey-headed Drongo, Fork-tailed backed Bush-shrike, Olive Drongo, Square-tailed Honeyguide, Greater Bush-Shrike, Orange- Duck, African Black Honeyguide, Lesser breasted Duck, White-faced Whistling Honeyguide, Scaly- Bustard, Black-bellied Eagle, African Crowned throated Buttonquail, Kurrichane Eagle, African Fish Hoopoe, African Buzzard, Common Eagle, Bateleur Hornbill, African -

Biodiversity Observations
Biodiversity Observations http://bo.adu.org.za An electronic journal published by the Animal Demography Unit at the University of Cape Town The scope of Biodiversity Observations consists of papers describing observations about biodiversity in general, including animals, plants, algae and fungi. This includes observations of behaviour, breeding and flowering patterns, distributions and range extensions, foraging, food, movement, measurements, habitat and colouration/plumage variations. Biotic interactions such as pollination, fruit dispersal, herbivory and predation fall within the scope, as well as the use of indigenous and exotic species by humans. Observations of naturalised plants and animals will also be considered. Biodiversity Observations will also publish a variety of other interesting or relevant biodiversity material: reports of projects and conferences, annotated checklists for a site or region, specialist bibliographies, book reviews and any other appropriate material. Further details and guidelines to authors are on this website. Paper Editor: Doug Harebottle BIRDS AND ANIMALS USING WEAVERS NESTS H. Dieter Oschadleus Recommended citation format: Oschadleus HD 2017. Birds and animals using weavers nests. Biodiversity Observations, Vol 8.28: 1-17 URL: http://bo.adu.org.za/content.php?id=323 Published online: 20 June 2017 Appendix added: 26 June 2017 – ISSN 2219-0341 – Biodiversity Observations 8.28: 1-17 1 PHOWN (PHOtos of Weaver Nests) Methods BIRDS AND ANIMALS USING WEAVERS NESTS The PHOWN database began in mid July 2010 and the data for this analysis was extracted up to 2 February 2017, providing 6.5 years of H. Dieter Oschadleus data collection. Records with Nest Use were marked so that they could be easily extracted. -

Species Diversity and Abundance of Wild Birds in Dagona-Waterfowl Sanctuary Borno State, Nigeria
African Journal of Environmental Science and Technology Vol. 5(10), pp. 855-866, October 2011 Available online at http://www.academicjournals.org/AJEST DOI: 10.5897/AJEST11.090 ISSN 1996-0786 ©2011 Academic Journals Full Length Research Paper Species diversity and abundance of wild birds in Dagona-Waterfowl Sanctuary Borno State, Nigeria Lameed, G. A. Department of Wildlife and Fisheries Management, Faculty of Agriculture and Forestry, University of Ibadan, Nigeria. E-mail: [email protected], [email protected]. Accepted 13 July, 2011 The study of bird species diversity and richness in Dagona-Waterfowl sanctuary was carried out in early wet and late dry seasons. This was to provide some information on the wild birds of the sanctuary. Dagona sanctuary is located within the Bade-Nguru wetland sector; it is one of the important bird areas strategized for the conservation of avifauna species in Sub-Sahara region, Nigeria. Line transect method was used to carry out birds’ survey at three different lake sites, namely: Gatsu (Site:1), Mariam (Site: 2) and Oxbow (Site: 3). The data were analyzed with the Kolmogorov- Smirnov test to determine the distribution level of the birds. The birds’ diversity was assessed using Shannon-Weiner diversity index. The results showed that bird species diversity was not equally distributed in the sites Site 2 had the highest diversity with an index of 2.74 compared to Site 1 with 1.84 and Site 3 with 1.62. Likewise, bird species diversity in the area was normally distributed birds’ abundance were significantly different (P<0.05) among the three sites. -

This Regulation Shall Enter Into Force on the Day Of
19 . 10 . 88 Official Journal of the European Communities No L 285 / 1 I (Acts whose publication is obligatory) COMMISSION REGULATION ( EEC ) No 3188 / 88 of 17 October 1988 amending Council Regulation ( EEC ) No 3626 / 82 on the implementation in the Community of the Convention on international trade in endangered species of wild fauna und flora THE COMMISSION OF THE EUROPEAN COMMUNITIES Whereas the measures provided for in this Regulation are in accordance with the opinion of the Committee on the Convention on International Trade in Endangered Species of Wild Fauna and Flora , Having regard to the Treaty establishing the European Economic Community , HAS ADOPTED THIS REGULATION : Having regard to Council Regulation ( EEC ) No 3626 / 82 Article 1 of 3 December 1982 on the implementation in the Corrimunity of the Convention on international trade in Appendix III of Annex A to Regulation ( EEC ) No 3626 / 82 endangered species of wild fauna and flora ( J ), as last is hereby replaced by the Annex to this Regulation . amended by Regulation ( EEC ) No 869 / 88 ( 2 ), and in particular Article 4 , thereof, Article 2 Whereas alterations were made to Appendix III to the This Regulation shall enter into force on the day of its publication in the Official Journal of the European Convention ; whereas Appendix III of Annex A to Communities . Regulation ( EEC ) No 3626 / 82 should now be amended to incorporate the amendments accepted by the Member States parties to the abovementioned Convention ; It shall apply from 21 September 1988 . This Regulation shall be binding in its entirety and directly applicable in all Member States . -

Ultimate Kenya
A pair of fantastic Sokoke Scops Owls. (DLV). All photos taken by DLV during the tour. ULTIMATE KENYA 1 – 20 / 25 APRIL 2017 LEADER: DANI LOPEZ-VELASCO Kenya lived up to its reputation of being one of the most diverse birding destinations on our planet. Once again, our Ultimate Kenya recorded a mind-boggling total of more than 750 species. This was despite the fact that we were prioritizing Kenyan specialities (a task in which we were extremely successful) rather than going all out for a huge list! 1 BirdQuest Tour Report: Ultimate Kenya www.birdquest-tours.com The first leg of our epic adventure saw us focusing on the Arabuko-Sokoke Forest where the birding is tough but the rewards are great. Over the course of the two and a half days our talented local guide helped us find all of the main specialities, with the exception of the difficult Clarke’s Weavers, which were presumably on their recently discovered breeding grounds in marshes to the north. Crested Guineafowl and Northern Carmine Bee-eater. We spent much time creeping along sandy tracks, gradually finding our targets one by one. We succeeded in getting great views of a number of skulkers, including a rather showy East Coast Akalat on our last afternoon, some reclusive Eastern Bearded Scrub Robins, a very obliging Red-tailed Ant Thrush and skulking Fischer’s and Tiny Greenbuls. Once in the Brachystegia we kept our eyes and ears open for roving flocks of flock-leader Retz’s and Chestnut-fronted Helmet Shrikes, and with these we found awkward Mombasa Woodpeckers and a single Green-backed Woodpecker, and a variety of smaller species including Black-headed Apalis, Green Barbet, Eastern Green Tinkerbird, dainty Little Yellow Flycatchers, Forest Batis, Pale Batis, cracking little Amani and Plain-backed Sunbirds and Dark-backed Weaver. -
Simplified-ORL-2019-5.1-Final.Pdf
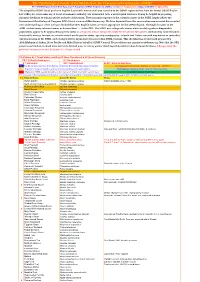
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part F: Simplified OSME Region List (SORL) version 5.1 August 2019. (Aligns with ORL 5.1 July 2019) The simplified OSME list of preferred English & scientific names of all taxa recorded in the OSME region derives from the formal OSME Region List (ORL); see www.osme.org. It is not a taxonomic authority, but is intended to be a useful quick reference. It may be helpful in preparing informal checklists or writing articles on birds of the region. The taxonomic sequence & the scientific names in the SORL largely follow the International Ornithological Congress (IOC) List at www.worldbirdnames.org. We have departed from this source when new research has revealed new understanding or when we have decided that other English names are more appropriate for the OSME Region. The English names in the SORL include many informal names as denoted thus '…' in the ORL. The SORL uses subspecific names where useful; eg where diagnosable populations appear to be approaching species status or are species whose subspecies might be elevated to full species (indicated by round brackets in scientific names); for now, we remain neutral on the precise status - species or subspecies - of such taxa. Future research may amend or contradict our presentation of the SORL; such changes will be incorporated in succeeding SORL versions. This checklist was devised and prepared by AbdulRahman al Sirhan, Steve Preddy and Mike Blair on behalf of OSME Council. Please address any queries to [email protected]. -

TOST 84(3).Indb
This article was downloaded by: [Queen's College], [David Lahti] On: 19 December 2013, At: 13:29 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Ostrich: Journal of African Ornithology Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/tost20 The sociality of nesting in Rüppell's Weaver Ploceus galbula and the Lesser Masked Weaver Ploceus intermedius in an Ethiopian acacia woodland David C Lahtia a Department of Biology, Queens College, City University of New York, Flushing, NY 11367, USA E-mail: Published online: 17 Dec 2013. To cite this article: David C Lahti (2013) The sociality of nesting in Rüppell's Weaver Ploceus galbula and the Lesser Masked Weaver Ploceus intermedius in an Ethiopian acacia woodland, Ostrich: Journal of African Ornithology, 84:3, 235-238 To link to this article: http://dx.doi.org/10.2989/00306525.2013.867548 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information.