Stimuli Characteristics and Psychophysical Requirements for Visual Training in Amblyopia: a Narrative Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contrast Sensitivity and Visual Acuity Among the Elderly
Contrast Sensitivity and Visual Acuity among the Elderly THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Mawada Osman Graduate Program in Vision Science The Ohio State University 2020 Master's Examination Committee: Angela M. Brown, PhD, Advisor Bradley E. Dougherty, OD, PhD Heidi A. Wagner, OD, MPH Copyright by Mawada Osman 2020 Abstract Purpose: To establish the clinical utility and strengthen the validity of the Ohio Contrast Cards (OCC), expand the use of the OCC in a healthy elderly population, and form a baseline dataset of patients to be compared to patients with dementia. Method: Participants ages 65 and over (N = 51) were recruited from the Ohio State University Primary Vision Care (PVC). We assessed the visual function of each patient using four visual tests which include: OCC, Pelli-Robson Chart (PR), Teller Acuity Cards (TAC) and Clear Chart. The contrast sensitivity tests (OCC and PR) were assessed twice, once by each tester. The PR contrast levels were also evaluated at two different distances 1 meter and 3 meters (0.50 meter if visual acuity worse than 6.0 cy/cm). Cognitive abilities were evaluated using the 6-Item Cognitive Impairment Test (6-CIT). Results: A significant effect of test was revealed (p < 0.005), in favor of OCC, yielding consistently higher average LogCS scores than PR, average difference of 0.412 LogCS. The PR and OCC revealed similar repeatability with 95% LoA of ± 0.28 log units and 95% LoA of ± 0.27 log units, respectively. -

The Hodgkin-Huxley Neuron Model for Motion Detection in Image Sequences Hayat Yedjour, Boudjelal Meftah, Dounia Yedjour, Olivier Lézoray
The Hodgkin-Huxley neuron model for motion detection in image sequences Hayat Yedjour, Boudjelal Meftah, Dounia Yedjour, Olivier Lézoray To cite this version: Hayat Yedjour, Boudjelal Meftah, Dounia Yedjour, Olivier Lézoray. The Hodgkin-Huxley neuron model for motion detection in image sequences. Neural Computing and Applications, Springer Verlag, In press, 10.1007/s00521-021-06446-0. hal-03331961 HAL Id: hal-03331961 https://hal.archives-ouvertes.fr/hal-03331961 Submitted on 2 Sep 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Noname manuscript No. (will be inserted by the editor) The Hodgkin{Huxley neuron model for motion detection in image sequences Hayat Yedjour · Boudjelal Meftah · Dounia Yedjour · Olivier L´ezoray Received: date / Accepted: date Abstract In this paper, we consider a biologically-inspired spiking neural network model for motion detection. The proposed model simulates the neu- rons' behavior in the cortical area MT to detect different kinds of motion in image sequences. We choose the conductance-based neuron model of the Hodgkin-Huxley to define MT cell responses. Based on the center-surround antagonism of MT receptive fields, we model the area MT by its great pro- portion of cells with directional selective responses. -

Visual Performance of Scleral Lenses and Their Impact on Quality of Life In
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE Visual performance of scleral lenses and their impact on quality of life in patients with irregular corneas Desempenho visual das lentes esclerais e seu impacto na qualidade de vida de pacientes com córneas irregulares Dilay Ozek1, Ozlem Evren Kemer1, Pinar Altiaylik2 1. Department of Ophthalmology, Ankara Numune Education and Research Hospital, Ankara, Turkey. 2. Department of Ophthalmology, Ufuk University Faculty of Medicine, Ankara, Turkey. ABSTRACT | Purpose: We aimed to evaluate the visual quality CCS with scleral contact lenses were 0.97 ± 0.12 (0.30-1.65), 1.16 performance of scleral contact lenses in patients with kerato- ± 0.51 (0.30-1.80), and 1.51 ± 0.25 (0.90-1.80), respectively. conus, pellucid marginal degeneration, and post-keratoplasty Significantly higher contrast sensitivity levels were recorded astigmatism, and their impact on quality of life. Methods: with scleral contact lenses compared with those recorded with We included 40 patients (58 eyes) with keratoconus, pellucid uncorrected contrast sensitivity and spectacle-corrected contrast marginal degeneration, and post-keratoplasty astigmatism who sensitivity (p<0.05). We found the National Eye Institute Visual were examined between October 2014 and June 2017 and Functioning Questionnaire overall score for patients with scleral fitted with scleral contact lenses in this study. Before fitting contact lens treatment to be significantly higher compared with scleral contact lenses, we noted refraction, uncorrected dis- that for patients with uncorrected sight (p<0.05). Conclusion: tance visual acuity, spectacle-corrected distance visual acuity, Scleral contact lenses are an effective alternative visual correction uncorrected contrast sensitivity, and spectacle-corrected contrast method for keratoconus, pellucid marginal degeneration, and sensitivity. -

Higher-Order Wavefront Aberration and Letter-Contrast Sensitivity In
Eye (2008) 22, 1488–1492 & 2008 Macmillan Publishers Limited All rights reserved 0950-222X/08 $32.00 www.nature.com/eye 1 1 2 2 CLINICAL STUDY Higher-order C Okamoto , F Okamoto , T Samejima , K Miyata and T Oshika1 wavefront aberration and letter-contrast sensitivity in keratoconus Abstract Keywords: keratoconus; wavefront aberration; higher-order aberration; contrast sensitivity; Aims To evaluate the relation between letter-contrast sensitivity higher-order aberration of the eye and contrast sensitivity function in eyes with keratoconus. Methods In 22 eyes of 14 patients with Introduction keratoconus (age 30.578.4 years, means7SD) and 26 eyes of 13 normal controls (age Keratoconus is a chronic, non-inflammatory 29.276.7 years), ocular higher-order wavefront disease of the cornea associated with aberration for a 6-mm pupil was measured progressive thinning and anterior protrusion of with the Hartmann-Schack aberrometer the central cornea.1,2 Irregular astigmatism is (KR-9000 PW, Topcon). The root mean square often the first and most apparent clinical finding (RMS) of third- and fourth-order Zernike in keratoconus, and this is evidenced by a coefficients was used to represent higher-order distortion of the corneal image as noted with aberrations. The letter-contrast sensitivity was the placido disc, retinoscope, keratometer, examined using the CSV-1000LV contrast chart keratoscope, and computerized (Vector Vision). videokeratograph. Previous studies using Results In the keratoconus group, the videokeratography have quantitatively 1 Department of letter-contrast sensitivity showed significant demonstrated that corneal irregular Ophthalmology, Institute of correlation with third-order (Spearman’s astigmatism is significantly greater in eyes with Clinical Medicine, University correlation coefficient ¼À0.736, 0.001) 3–6 of Tsukuba, Ibaraki, Japan r Po keratoconus than in normal eyes. -

Capture of Stereopsis and Apparent Motion by Illusory Contours
Perception & Psychophysics 1986, 39 (5), 361-373 Capture of stereopsis and apparent motion by illusory contours V. S. RAMACHANDRAN University of California, San Diego, La Jolla, California The removal of right-angle sectors from four circular black disks created the impression of a white "subjective" square with its four corners occluding the disks ("illusory contours"). The dis play in one eye was identical to that in the other except that for one eye a horizontal disparity was introduced between the edges of the cut sectors so that an illusory white square floated out in front of the paper. When a "template" ofthis stereogram was superimposed on a vertical square wave grating (6 cycles/deg) the illusory square pulled the corresponding lines of grating forward even though the grating was at zero disparity. (Capture was not observed if the template was superimposed on horizontal gratings; the grating remained flush with the background). Analo gous effects were observed in apparent motion ("motion capture"). Removal of sectors from disks in an appropriately timed sequence generated apparent motion of an illusory square. When a template of this display was superimposed on a grating, the lines appeared to move vividly with the square even though they were physically stationary. It was concluded that segmentation of the visual scene into surfaces and objects can profoundly influence the early visual processing of stereopsis and apparent motion. However, there are interesting differences between stereo cap ture and motion capture, which probably reflect differences in natural constraints. The implica tions of these findings for computational models of vision are discussed. -

MARY BALDWIN COLLEGE 2003–2004 ACADEMIC CATALOG for Undergraduate and Graduate Programs Vol
MARY BALDWIN COLLEGE 2003–2004 ACADEMIC CATALOG for Undergraduate and Graduate Programs Vol. 50 JUNE 2003 Mary Baldwin College Staunton, VA 24401 www.mbc.edu CONTACT INFORMATION Academic Affairs M.Litt./MFA in Shakespeare REGIONAL CENTERS Dean of the College 540-887-7237 540-887-7030 540-887-7058 MBC/BRCC Adult Degree Program [email protected] Blue Ridge Community College Admissions P.O. Box 80 1-800-468-2262 Office of the President Weyers Cave, VA 24486 540-887-7019 540-887-7026 540-453-2345 [email protected] [email protected] Program for the Adult Degree Program Exceptionally Gifted Mary Baldwin College at PVCC ADP House on Campus 540-887-7039 Piedmont Virginia 1-800-822-2460 [email protected] Community College 540-887-7003 501 College Dr. [email protected] Public Information Charlottesville, VA 22902-7589 See Regional Centers College Relations 434-961-5422 540-887-7009 [email protected] Alumnae/i Activities [email protected] 1-800-763-7359 Mary Baldwin College 540-887-7007 Safety and Security in Northern Virginia [email protected] 540-887-7000 2 Pidgeon Hill Dr., Suite 240 Sterling, VA 20165 Bookstore Rosemarie Sena Center 703-444-2524 540-887-7264 for Student Life and [email protected] [email protected] Career Development 540-887-7221 Mary Baldwin College in Richmond Business Office [email protected] 1801 Libbie Ave. 540-887-7175 Richmond, VA 23226 Student Records and Transcripts 804-282-9111 Financial Aid and Student Office of the Registrar [email protected] Campus Employment 540-887-7071 1-800-468-2262 Mary Baldwin College in Roanoke 540-887-7022 Switchboard 108 N. -

Unsupervised Learning of a Hierarchical Spiking Neural Network for Optical Flow Estimation: from Events to Global Motion Perception
IEEE TRANSACTIONS ON PATTERN ANALYSIS AND MACHINE INTELLIGENCE, 2019 1 Unsupervised Learning of a Hierarchical Spiking Neural Network for Optical Flow Estimation: From Events to Global Motion Perception Federico Paredes-Valles´ , Kirk Y. W. Scheper , and Guido C. H. E. de Croon , Member, IEEE Abstract—The combination of spiking neural networks and event-based vision sensors holds the potential of highly efficient and high-bandwidth optical flow estimation. This paper presents the first hierarchical spiking architecture in which motion (direction and speed) selectivity emerges in an unsupervised fashion from the raw stimuli generated with an event-based camera. A novel adaptive neuron model and stable spike-timing-dependent plasticity formulation are at the core of this neural network governing its spike-based processing and learning, respectively. After convergence, the neural architecture exhibits the main properties of biological visual motion systems, namely feature extraction and local and global motion perception. Convolutional layers with input synapses characterized by single and multiple transmission delays are employed for feature and local motion perception, respectively; while global motion selectivity emerges in a final fully-connected layer. The proposed solution is validated using synthetic and real event sequences. Along with this paper, we provide the cuSNN library, a framework that enables GPU-accelerated simulations of large-scale spiking neural networks. Source code and samples are available at https://github.com/tudelft/cuSNN. Index Terms—Event-based vision, feature extraction, motion detection, neural nets, neuromorphic computing, unsupervised learning F 1 INTRODUCTION HENEVER an animal endowed with a visual system interconnected cells for motion estimation, among other W navigates through an environment, turns its gaze, or tasks. -

Contrast Sensitivity and Visual Acuity in Low-Vision Students Thesis
Contrast Sensitivity and Visual Acuity in Low-Vision Students Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Steve Murimi Mathenge Njeru, BS Graduate Program in Vision Science The Ohio State University 2020 Thesis Committee Angela M. Brown, PhD, Advisor Bradley E. Dougherty, OD, PhD Deyue Yu, PhD Copyrighted by Steve Murimi Mathenge Njeru 2020 Abstract Purpose: This study primarily compared the test-retest reliability of the Pelli-Robson chart (PR) and Ohio Contrast Cards (OCC) amongst testers. The secondary goal of this study was to examine the impact on contrast sensitivity if the testing distance for the Pelli-Robson chart were to be changed. An additional goal was to evaluate the relationship between visual acuity (VA) and contrast sensitivity (CS) when using letter-based charts and grating cards. Methods: Thirty low-vision students were tested, ranging from 7-20 years old. Each student was tested with both VA and CS tests in randomized order, which included: the Bailey- Lovie chart (BL), Pelli-Robson chart, Teller Acuity Cards (TAC), and Ohio Contrast Cards. Each student repeated both the PR chart and OCC in separate rooms, but neither the BL chart nor TAC was repeated. The PR chart was also tested at closer testing distance, based on the student’s logMAR acuity from the BL chart. For the letter charts, a letter-by-letter scoring method was used. For grating cards, these were both scored as preferential looking tests. Results: The Limits of Agreement for the OCC and PR chart were +/- 0.451 and +/- 0.536, respectively. -
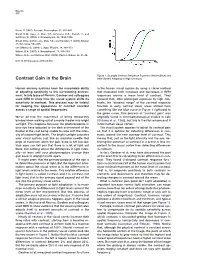
Contrast Gain in the Brain After (Green) Adapting to High Contrasts
Neuron 476 Rozin, P. (1982). Percept. Psychophys. 31, 397–401. Small, D.M., Voss, J., Mak, Y.E., Simmons, K.B., Parrish, T., and Gitelman, D. (2004). J. Neurophysiol. 92, 1892–1903. Small, D.M., Gerber, J.C., Mak, Y.E., and Hummel, T. (2005). Neuron 47, this issue, 593–605. von Békésy, G. (1964). J. Appl. Physiol. 19, 369–373. Wilson, D.A. (1997). J. Neurophysiol. 78, 160–169. Wilson, D.A., and Sullivan, R.M. (1999). Physiol. Behav. 66, 41–44. DOI 10.1016/j.neuron.2005.08.002 Figure 1. Example Contrast Response Functions Before (Blue) and Contrast Gain in the Brain After (Green) Adapting to High Contrasts Human sensory systems have the remarkable ability in the human visual system by using a clever method of adjusting sensitivity to the surrounding environ- that measured both increases and decreases in fMRI ment. In this issue of Neuron, Gardner and colleagues responses around a mean level of contrast. They used fMRI to show how the visual system shifts its showed that, after prolonged exposure to high con- sensitivity to contrast. This process may be helpful trasts, the “dynamic range” of the contrast response for keeping the appearance of contrast constant function in early cortical visual areas shifted from across a range of spatial frequencies. something like the blue curve in Figure 1 rightward to the green curve. This process of “contrast gain” was We’ve all had the experience of being temporarily originally found in electrophysiological studies in cats blinded when walking out of a movie theater into bright (Ohzawa et al., 1985), but this is the first evidence of it sunlight. -

Contrast Sensitivity and Acuity Relationship in Strabismic and Anisometropic Amblyopia
Br J Ophthalmol: first published as 10.1136/bjo.72.1.44 on 1 January 1988. Downloaded from British Journal of Ophthalmology, 1988, 72, 44-49 Contrast sensitivity and acuity relationship in strabismic and anisometropic amblyopia M ABRAHAMSSON AND J SJOSTRAND From the Department of Ophthalmology, University of Goteborg, Sahlgren's Hospital S-413 45 Goteborg, Sweden SUMMARY The contrast sensitivity function (CSF) and visual acuity were determined in children and adults with unilateral amblyopia due to strabismus or anisometropia with central fixation. The preschool children were examined repeatedly during occlusion treatment. All amblyopes had CSF deficits. The CSF was characterised by its peak value (the maximal sensitivity, Smax, and the spatial frequency at which Smax occurs, Frmax) calculated by a single peak least-square regression method. The two amblyopic groups showed discrepancies in relationship of both Smax and Frmax versus visual acuity both initially and during treatment. The strabismic cases had a more marked visual acuity deficit in relation to the contrast sensitivity losses, whereas these parameters are affected similarly in anisometropic amblyopes. The relationship between recovery of visual acuity and CSF during the initial month of occlusion treatment was of prognostic significance for the outcome of visual acuity improvement. copyright. Amblyopia is defined as an optically uncorrectable receives an input with deprived form and contour. loss of vision, usually monocular, without demon- There is no evidence that a strabismic eye receives a strable pathology in the posterior pole of the eye. blurred image, whereas several studies indicate that This condition develops in early childhood and it abnormal binocular interaction is present in http://bjo.bmj.com/ affects up to 5% of the population. -

Seven Myths on Crowding1
Myths on Crowding_39.doc Seven myths on crowding1 Hans Strasburger, Ludwig-Maximilians-Universität, München, Germany For submission to i-Perception Abstract Crowding has become a hot topic in vision research and some fundamentals are now widely agreed upon. For the classical crowding task one would likely agree with the following statements. (1) Bouma’s law can be succinctly stated as saying that critical distance for crowding is about half the target’s eccentricity. (2) Crowding is predominantly a peripheral phenomenon. (3) Peripheral vision extends to at most 90° eccentricity. (4) Crowding increases strongly and linearly with eccentricity (as does the minimal angle of resolution, MAR). (5) Crowding is asymmetric as Bouma (1970) has shown. For that inner-outer asymmetry, the peripheral flanker has more effect. (6) Critical crowding distance corresponds to a constant cortical distance in primary visual areas like V1. (7) Except for Bouma’s (1970) paper, crowding research mostly started in the 2000s. I propose the answer is ‘not really’ to these assertions. So should we care? I think we should, before we write the textbooks for the next generation. Keywords: Crowding, Psychophysics, Perception, Reading, Visual acuity, Peripheral vision, Fovea, Asymmetries, Sensory systems, Cortical map, Vision science, Visual field. Introduction In 1962, the ophthalmologists James Stuart and Hermann Burian published a study on amblyopia where they adopted a nice and clear term, crowding, to describe why standard acuity test charts are mostly unsuitable for amblyopic subjects: On most standard charts, as ophthalmologists and optometrists knew, optotypes on a line are too closely spaced for valid assessment of acuity in all cases, such that in particular amblyopic subjects (and young children) may receive too low an acuity score. -

Human Visual Motion Perception Shows Hallmarks of Bayesian Structural Inference Sichao Yang1,2,6*, Johannes Bill3,4,6*, Jan Drugowitsch3,5,7 & Samuel J
www.nature.com/scientificreports OPEN Human visual motion perception shows hallmarks of Bayesian structural inference Sichao Yang1,2,6*, Johannes Bill3,4,6*, Jan Drugowitsch3,5,7 & Samuel J. Gershman4,5,7 Motion relations in visual scenes carry an abundance of behaviorally relevant information, but little is known about how humans identify the structure underlying a scene’s motion in the frst place. We studied the computations governing human motion structure identifcation in two psychophysics experiments and found that perception of motion relations showed hallmarks of Bayesian structural inference. At the heart of our research lies a tractable task design that enabled us to reveal the signatures of probabilistic reasoning about latent structure. We found that a choice model based on the task’s Bayesian ideal observer accurately matched many facets of human structural inference, including task performance, perceptual error patterns, single-trial responses, participant-specifc diferences, and subjective decision confdence—especially, when motion scenes were ambiguous and when object motion was hierarchically nested within other moving reference frames. Our work can guide future neuroscience experiments to reveal the neural mechanisms underlying higher-level visual motion perception. Motion relations in visual scenes are a rich source of information for our brains to make sense of our environ- ment. We group coherently fying birds together to form a fock, predict the future trajectory of cars from the trafc fow, and infer our own velocity from a high-dimensional stream of retinal input by decomposing self- motion and object motion in the scene. We refer to these relations between velocities as motion structure.