The Impact of Menopause on Multiple Sclerosis: a Multicentre, Retrospective, Observational Study Authors: D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Libretto Orari Linea Z301 in Vigore Dal 01-04-19
I I I LIBRETTO ORARI I LINEA Z301 IN VIGORE DAL 01-04-19 Per informazioni: NET NordEst Trasporti Num.Verde 800-90.51.50 ore 7.30-19.30 tutti i giorni Z301 Z301-As MILANO - BERGAMO ................................................................ 4 Z301-Di BERGAMO - MILANO ................................................................. 7 Trezzo A4 Indice Fermate Via Battisti AGRATE BRIANZA Agrate A4 BERGAMO Bergamo Autostazione Bergamo Autostazione (scarico) Via Autostrada Via Carnovali BREMBATE Capriate A4 CAVENAGO DI BRIANZA Cavenago A4 CINISELLO BALSAMO V.le Brianza COLOGNO MONZESE Cologno Nord Cologno Nord M2 CORMANO Cormano A4 DALMINE Dalmine A4 Fermata A4 MILANO Laghi Lampugnano Lampugnano M1 Sarca NOVATE MILANESE Novate Milanese Novate Milanese A4 SAN DONATO MILANESE De Gasperi Maritano SESTO SAN GIOVANNI Gramsci M.te Grappa Sesto F.S. Sesto Rondo' TREZZO SULL'ADDA Piazzale Primo Maggio Z301-As MILANO - BERGAMO lunedì-venerdì Note z2 MILANO Lampugnano 05.40 06.15 06.45 06.45 CORMANO uscita A4 05.53 06.28 06.58 06.58 SARCA/RABOLINI 05.20 SESTO S.G. M1 05.35 06.45 07.00 SESTO S.G. Cornaggia 05.41 06.51 07.06 SAN DONATO SNAM COLOGNO NORD M2 AGRATE casello A4 05.49 06.05 06.40 06.59 07.10 07.10 07.14 CAVENAGO casello A4 05.52 06.08 06.43 07.02 07.13 07.13 07.17 TREZZO Biffi 04.50 05.10 05.30 06.00 06.15 06.25 06.35 06.55 07.00 07.00 TREZZO casello A4 05.01 05.21 05.41 05.58 06.11 06.14 06.26 06.36 06.46 06.49 07.06 07.08 07.11 07.11 07.19 07.19 07.23 CAPRIATE casello A4 05.03 05.23 05.43 06.00 06.13 06.16 06.28 06.38 06.48 06.51 07.08 07.10 07.13 07.13 07.21 07.21 07.25 DALMINE casello A4 05.08 05.28 05.48 06.05 06.18 06.21 06.33 06.43 06.53 06.56 07.13 07.15 07.18 07.18 07.26 07.26 07.30 BERGAMO Autostazione 05.16 05.36 05.56 06.13 06.26 06.29 06.42 06.52 07.02 07.05 07.22 07.24 07.27 07.27 07.35 07.35 07.39 z2) Eccetto giorni di chiusura delle scuole lunedì-venerdì Note z2 MILANO Lampugnano 07.15 07.35 07.55 08.15 08.35 09.10 CORMANO uscita A4 07.28 07.48 08.08 08.28 08.48 09.23 SARCA/RABOLINI 06.55 08.00 08.35 09.35 SESTO S.G. -

Environmental Suppliers Customer and Citizens Institutions and Communities Susainability Report - 2012 1 Index
Sustainability Report 2012 employees shareholders and investors environmental suppliers customer and citizens institutions and communities Susainability report - 2012 1 Index Index Letter to stakeholders 4 Introduction and note on methodology 6 The A2A Group’s Sustainability Report 6 Methodology 6 Scoping logic 7 Materiality 9 1 The A2A group 10 1.1 Size of the organisation and markets served 14 1.2 Companies outside the consolidation scope 15 2 Strategies and policies for sustainability 17 2.1 Mission and vision 18 2.2 Commitments and objectives for the future 18 2.3 The 2013-2015 Sustainability Plan and key indicators/results achieved in 2012 19 2.4 Partnerships and awards for sustainability 25 2.5 Corporate governance 27 Corporate governance bodies 27 Corporate governance tools 34 Regulatory framework 35 2.6 Stakeholder chart and engagement initiatives 37 3 Economic responsibility 41 3.1 The Group’s 2012 results 43 3.2 Formation of Value Added 43 3.3 Distribution of Net Global Value Added 45 3.4 Capital expenditure 46 3.5 Shareholders and Investors 47 Composition of share capital 47 A2A in the stock exchange indices 48 A2A in the sustainability ratings 49 2 Susainability report - 2012 Index Investing in A2A: sustainability as a valuation project 49 Relations with shareholders and investors 52 3.6 Tables: the numbers of the A2A Group 55 4 Environmental responsibility 57 4.1 Managing the environment 58 Environmental policy 59 Environmental management system 59 Environmental risk management 60 Environmental accounting 61 Research and -

Assistenza Domiciliare Integrata ADI Consultori Familiari E Consultori Adolescenti Servizi E Progetti Rivolti Alle Persone Fragili
1 Gli Ospedali Unità Operative Poliambulatori Ospedalieri 1 Presidio Vizzolo Predabissi – Cassano d’Adda Ospedale “Predabissi” Via Pandina 1 – Vizzolo Predabissi Anatomia Patologica Anestesia Rianimazione – Terapia Intensiva Attività Cure Subacuti Cardiologia - UTIC Chirurgia Generale Day Hospital Day Surgery Medicina Generale Nefrologia e Dialisi Neurologia - Stroke Unit Oculistica Oncologia Ortopedia Traumatologia Ostetricia Ginecologia Otorinolaringoiatria Pediatria - Nido Pronto Soccorso Generale Pronto Soccorso Pediatrico Psichiatria - SPDC Urologia Ospedale “Zappatoni” Dietologia e Nutrizione Clinica Via Q. di Vona 41 – Cassano Endoscopia Digestiva d’Adda Farmacia Riabilitazione specialistica: Laboratorio Analisi – SIMT Cardiologica Poliambulatorio Pneumologica Radiologia Neuromotoria Terapia Fisica CAL Blocco Operatorio Centrale di Sterilizzazione Terapia Fisica Piccola Guida al Ricovero Piccola Guida al Ricovero 2 OSPEDALE “PREDABISSI” VIZZOLO PREDABISSI AMBULATORI SPECIALISTICI Allergologia Angiologia Cardiologia Chirurgia Chirurgia Plastica Dermatologia Diabetologia Endocrinologia Nefrologia Neurochirurgia Neurologia Medicina Oculistica Oftalmologia Pediatria Oncologia Ortopedia Ostetricia Ginecologia Otorinolaringoiatria Pediatria Pneumologia Reumatologia Terapia del dolore Urologia OSPEDALE “ZAPPATONI – CASSANO D’ADDA AMBULATORI SPECIALISTICI Allergologia Cardiologia Cure Fisiche Fisiatria Geriatria Oculistica Ortopedia Otorinolaringoiatria Pneumatologia 3 Presidio Cernusco sul Naviglio – Vaprio d’Adda Ospedale “Uboldo” -

Carta Dei Servizi Cdi Legnano
DocuSign Envelope ID: 419ECCBF-D253-4E31-9514-33674ACB5835 CARTA DEI SERVIZI CDI LEGNANO CORSO ITALIA 32 LEGNANO Punto Prelievi SSN (MI) Direttore Sanitario: prof. A. Casasco www.cdi.it Direttore Laboratorio: dr. F. Ferrara DocuSign Envelope ID: 419ECCBF-D253-4E31-9514-33674ACB5835 CHI SIAMO 2 CDI Centro Diagnostico Italiano è un’azienda sanitaria presente sul territorio milanese dal 1975 interamente dedicata al servizio della salute: prevenzione, diagnosi, terapia, supportate da un elevato standard tecnologico e dal costante aggiornamento delle linee guida internazionali. CDI eroga prestazioni sanitarie sia in regime di accreditamento con il Servizio Sanitario Nazionale sia in regime privato e fondi assicurativi. La nostra mission Consolidare la leadership di CDI attraverso l’offerta alla comunità della più ampia gamma di servizi e prestazioni di prevenzione, diagnosi e terapia, erogabili ambulatorialmente o in day hospital, nella costante ricerca del miglioramento continuo della qualità del servizio e dell’eccellenza tecnica. La nostra vision Il laboratorio CDI eccelle nella prestazione tecnica attraverso il miglioramento e l’innovazione, estende la gamma dei servizi, migliora l’offerta logistica e semplifica i processi di erogazione del servizio. Tali obbiettivi si realizzano attraverso il Piano strategico definito dalla Direzione. L’Unità Produttiva del Laboratorio introduce e sviluppa nuove indagini diagnostiche all’interno di percorsi diagnostico-terapeutici strategici. Il reparto offre un servizio completo e rapido ai suoi clienti interni ed esterni, per cui ottimizza e migliora continuamente l’organizzazione in riferimento ad attività per grandi volumi. L’imaging L’Unità Produttiva eroga servizi ad alta specializzazione attraverso l’integrazione tra le tecnologie avanzate, le competenze evolute e mediante la disponibilità e l’uso di infrastrutture e software dedicati nella generale evoluzione verso moderni sistemi di Imaging senza pellicola. -

Adp Pia Navigli Progetto Integrato D
RESTAURO CONSERVATIVO E MESSA IN SICUREZZA DIGHE DEL PANPERDUTO 2° LOTTO 1° STRALCIO COMUNE DI SOMMA LOMBARDO COMUNE DI SOMMA LOMBARDO V9: € 5.504.000 V10 - OPERA DI PRESA PRINCIPALE: € 1.594.000 ADP PIA NAVIGLI V11 - CONCA DI NAVIGAZIONE MADDALENA 1: € 800.000 ROBBIATE V12 - CONCA DI NAVIGAZIONE MADDALENA 2: € 950.000 NAVIGLIO PADERNO PADERNO D`ADDA !n SOMMA LOMBARDO PROGETTO INTEGRATO D'AREA !² INSTALLAZIONE PONTILI MOBILI !² !? COMUNI DI ABBIATEGRASSO, FIUME TICINO !² SISTEMAZIONE CANALE DI ACCESSO ALLA CONCA TREZZO SULL'ADDA E VAPRIO D'ADDA MADDALENA 1, MANDRACCHIO E ORMEGGI N3: € 100.000 CORNATE D`ADDA COMUNE DI SOMMA LOMBARDO RECUPERO ROBINIETI - LOC. FORNACI !² V14: € 785.000 COMUNE DI GARBAGNATE MILANESE PG2: € 116.000 PERCORSO BOTANICO PARCO OSPEDALE TREZZO SULL`ADDA !m COMUNI DI GARBAGNATE MILANESE E SENAGO RIQUALIFICAZIONE GIARDINO CASA RESTAURO CASELLO IDRAULICO PG1: € 294.000 REALIZZAZIONE ITINERARIO CICLABILE CUSTODE DELLE ACQUE COMUNE DI GARBAGNATE MILANESE COMUNI DI TREZZO SULL'ADDA E VAPRIO D'ADDA !x COMUNE DI VAPRIO D'ADDA !¯ V13: € 494.000 PAD1: € 700.000 !G PAD2: € 220.000 ALLESTIMENTO MUSEALE LONATE POZZOLO PAD3: € 77.000 RIPRISTINO DIGA POIRET MUSEO ED EMEROTECA DELLE ACQUE !Ï!Ï !?!x COMUNE DI TURBIGO !? SENAGO VAPRIO D`ADDA !Ú COMUNE DI CASTANO PRIMO V18a: € 4.000.000 V4: € 435.000 GARBAGNATE MILANESE !Ï!m NOSATE !? CANALE VILLORESI NAVIGLIO MARTESANA CONCHE E OPERE ACCESSORIE CASTANO PRIMO COMUNI DI TURBIGO E NOSATE !m!G ADDANDO IN BICI - REALIZZAZIONE V18b: € 2.500.000 !m PISTA CICLABILE !n TURBIGO COMUNE DI MAGENTA, -

Sede Inps Cod.Sede Regione Prov Cap Comune
SEDE INPS COD.SEDE REGIONE PROV CAP COMUNE BERGAMO 1200 LOMBARDIA BG 24121 BERGAMO CLUSONE 1291 LOMBARDIA BG 24023 CLUSONE GRUMELLO DEL MONTE 1294 LOMBARDIA BG 24064 GRUMELLO DEL MONTE ROMANO DI LOMBARDIA 1295 LOMBARDIA BG 24058 ROMANO DI LOMBARDIA TERNO D'ISOLA 1293 LOMBARDIA BG 24030 TERNO D'ISOLA TREVIGLIO 1290 LOMBARDIA BG 24047 TREVIGLIO ZOGNO 1292 LOMBARDIA BG 24019 ZOGNO BRENO 1590 LOMBARDIA BS 25043 BRENO BRESCIA 1500 LOMBARDIA BS 25123 BRESCIA CHIARI 1595 LOMBARDIA BS 25032 CHIARI DESENZANO DEL GARDA 1593 LOMBARDIA BS 25015 DESENZANO DEL GARDA ISEO 1597 LOMBARDIA BS 25049 ISEO MANERBIO 1594 LOMBARDIA BS 25025 MANERBIO MONTICHIARI 1596 LOMBARDIA BS 25018 MONTICHIARI SAREZZO 1592 LOMBARDIA BS 25068 SAREZZO VILLANUOVA SUL CLISI 1591 LOMBARDIA BS 25089 VILLANUOVA SUL CLISI CANTU' 2490 LOMBARDIA CO 22063 CANTU' COMO 2400 LOMBARDIA CO 22100 COMO ERBA 2491 LOMBARDIA CO 22036 ERBA MENAGGIO (INATTIVA) 2492 LOMBARDIA CO 22017 MENAGGIO CASALMAGGIORE 2691 LOMBARDIA CR 26041 CASALMAGGIORE CREMA 2690 LOMBARDIA CR 26013 CREMA CREMONA 2600 LOMBARDIA CR 26100 CREMONA LECCO 2401 LOMBARDIA LC 23900 LECCO MERATE 2493 LOMBARDIA LC 23807 MERATE CODOGNO 4992 LOMBARDIA LO 26845 CODOGNO LODI 4927 LOMBARDIA LO 26900 LODI SANT'ANGELO LODIGIANO 4977 LOMBARDIA LO 26866 SANT'ANGELO LODIGIANO CARATE BRIANZA (INATTIVA) 4978 LOMBARDIA MB 20841 CARATE BRIANZA CESANO MADERNO 4996 LOMBARDIA MB 20811 CESANO MADERNO DESIO 4995 LOMBARDIA MB 20832 DESIO MONZA 4901 LOMBARDIA MB 20052 MONZA SEDE INPS COD.SEDE REGIONE PROV CAP COMUNE SEREGNO 4909 LOMBARDIA MB 20831 SEREGNO -

Digestive and Liver Disease an International Journal of Gastroenterology and Hepatology Vol
VOLUME 50 SUPPLEMENT 3 October 2018 ISSN 1590-8658 Abstracts of the A.I.S.F. - Italian Association for the Study of the Liver - Monothematic Conference “Th e autoimmune diseases of the liver and the biliary system” Bologna, October 4th- 5th, 2018 Digestive and Liver Disease An International Journal of Gastroenterology and Hepatology Vol. 50 Supplement 3 (2018) Official Journal of: Italian Association for Hospital Gastroenterologists and Digestive Endoscopists Italian Society of Gastroenterology (SIGE) (AIGO) Italian Society of Pediatric Gastroenterology and Hepatology (SIGENP) Italian Association for the Study of the Liver (AISF) Italian Group for the Study of Infl ammatory Bowel Disease (IG-IBD) Italian Association for the Study of the Pancreas (AISP) Fédération Francophone de Cancérologie Digestive (FFCD) Italian Association for Digestive Endoscopy (SIED) Editors Emeriti Editor-in-Chief Managing Editor Mario Angelico, Rome, Italy Roberto de Franchis, Milan, Italy Silvia Malosio, Milan, Italy Gabriele Bianchi-Porro, Milan, Italy Co-Editors Editorial Assistant Savino Bruno, Milan, Italy Brenda Dionisi, Milan, Italy Silvia Fargion, Milan, Italy Maurizio Vecchi, Milan, Italy ASSOCIATE EDITORS DESIGNATED BY THE AFFILIATED SOCIETIES Carlo Agostoni (SIGENP), Milan, Italy Edoardo Giannini (AISF), Genoa, Italy Franco Radaelli (SIED), Como, Italy Carolina Ciacci (SIGE), Naples, Italy Gioacchino Leandro (AIGO), Castellana Grotte, Italy Fernando Rizzello (IG-IBD), Bologna, Italy Raffaele Pezzilli (AISP), Bologna, Italy SECTION EDITORS Basic Science -

Lista Sezione N. 3
Elezione del Consiglio Metropolitano di Milano Domenica 9 ottobre 2016 Lista aventi diritto al voto SEZIONE N. 3 Fascia D) Comuni con popolazione superiore a 10.000 e fino a 30.000 abitanti – SCHEDA ROSSA Data di Fascia Comune Cognome Nome Sesso Luogo di nascita Colore scheda nascita 1 D ARESE PALESTRA MICHELA F 19/03/1973 MILANO (MI) ROSSO 2 D ARESE BALSAMO LORIS M 01/07/1994 RHO (MI) ROSSO 3 D ARESE BELLUNATO TITO FLAVIO M 31/08/1974 MORBEGNO (SO) ROSSO 4 D ARESE BETTINARDI GIUSEPPE M 28/05/1960 BOLLATE (MI) ROSSO 5 D ARESE BURONI EDOARDO M 13/04/1981 BOLLATE (MI) ROSSO 6 D ARESE CASTELLI ANTONIO M 07/02/1968 AGRIGENTO (AG) ROSSO 7 D ARESE CATTANEO SERGIO M 23/06/1953 MILANO (MI) ROSSO 8 D ARESE CEREA VERONICA F 06/01/1975 BOLLATE (MI) ROSSO 9 D ARESE GIUDICI CARLO M 24/07/1943 ARESE (MI) ROSSO 10 D ARESE MIRAGOLI ANDREA M 13/02/1989 MILANO (MI) ROSSO 11 D ARESE MURATORI LUIGI M 20/06/1965 LECCE (LE) ROSSO 12 D ARESE NUVOLI LUCA M 28/08/1988 MELZO (MI) ROSSO 13 D ARESE PANDOLFI PAOLA F 07/10/1971 MILANO (MI) ROSSO 14 D ARESE PERGOLI ILIA F 21/02/1983 DESIO (MI) ROSSO 15 D ARESE PIOVESAN UMBERTO M 02/11/1967 BOLLATE (MI) ROSSO 16 D ARESE TONIOLO PAOLA F 21/05/1955 MARINGÀ, PARANÀ (BRASILE) ROSSO 17 D ARESE VARRI CHIARA MARIA F 14/05/1978 MILANO (MI) ROSSO 18 D ARLUNO AGOLLI MORENO M 27/08/1969 RHO (MI) ROSSO 19 D ARLUNO ALFIERI LUIGI M 17/10/1961 ARLUNO (MI) ROSSO 20 D ARLUNO BASSANI OMBRETTA F 18/06/1981 CUGGIONO (MI) ROSSO 21 D ARLUNO BERRA ANNA F 23/05/1973 MAGENTA (MI) ROSSO 22 D ARLUNO BONAZZOLI IGOR M 25/03/1979 MAGENTA (MI) ROSSO -

Distretti Veterinari Distretti Veterinari
DISTRETTI VETERINARI DISTRETTO VETERINARIO n.1 DISTRETTO VETERINARIO n.2 DISTRETTO VETERINARIO n.3 Comprende gli ambiti territoriali delle aree dei Distretti 1 Comprende gli ambiti territoriali delle aree dei Distretti 4 Comprende gli ambiti territoriali delle aree dei Distretti 6 (Garbagnate), 2 (Rho) e 3 (Corsico) (Legnano) e 5 (Castano Primo) (Magenta) e 7 (Abbiategrasso) Elenco comuni Distretto Veterinario n. 1: Elenco comuni Distretto Veterinario n. 2: Elenco comuni Distretto Veterinario n. 3: Arese - Assago - Baranzate - Bollate - Buccinasco - Cesano Bo- Arconate - Bernate Ticino - Buscate - Busto Garolfo - Canegrate - Abbiategrasso - Albairate - Arluno - Bareggio - Besate - Boffalora scone - Cesate - Cornaredo - Corsico - Cusago -Garbagnate Mila- Castano Primo - Cerro Maggiore - Cuggiono - Dairago - Inveruno sopra Ticino - Bubbiano - Calvignasco - Casorezzo - Cassinetta di nese - Lainate - Novate Milanese - Paderno Dugnano - Pero - Legnano - Magnano - Nerviano - Nosate - Parabiago - Rescaldina - Lugagnano - Cisliano - Corbetta - Gaggiano - Gudo Visconti - Ma- Pogliano Milanese - Pregnana Milanese - Rho - Senago - Settimo Robecchetto con Induco - San Giorgio su Legnano - Turbigo - San genta - Marcallo con Casone - Mesero - Moribondo - Motta Visconti - Ossona - Ozzero - Robecco sul Naviglio - Rosate - S. Stefano Milanese - Solaro - Trezzano sul Naviglio - Vanzago - Vittore Olona - Vanzaghello - Villa Cortese Ticino - Sedriano - Vermezzo - Vittuone - Zelo Surrigone Sede e Direzione: Tel. 02 93923330/5 Sede e Direzione: Tel. 0331 498529 -

Elenco Dei Laboratori Per Il Test Sierologico
Elenco dei laboratori per il test sierologico ENTE STRUTTURA INDIRIZZO POLIDIAGNOSTICO AFFIDEA LOMBARDIA S.R.L. VIA BRERA, 23 - 20010 - CORNAREDO (MI) MONTESANTO ASST CENTRO SPECIALISTICO Istituto Ortopedico ORTOPEDICO TRAUMATOLOGICO PIAZZA CARDINAL FERRARI, 1 - 20100 - MILANO Gaetano Pini GAETANO PINI/CTO Ospedale Civico - VIALE MARCONI, 1 - 26845 - CODOGNO (LO) Codogno ASST DI LODI Presidio Ospedaliero LARGO DONATORI DI SANGUE, SNC 26900 - LODI di Lodi PRESIDIO OSPEDALIERO VIA CASTELVETRO, 22 - 20100 - MILANO VITTORE BUZZI Ospedale LUIGI VIA G.B. GRASSI, 74 - 20100 - MILANO SACCO ASST FATEBENEFRATELLI SACCO Presidio Ospedaliero VIA MACEDONIO MELLONI, 52 - 20100 - MILANO Macedonio Melloni Presidio Fatebenefratelli e CORSO DI PORTA NUOVA, 23 - 20100 - MILANO Oftalmico Presidio Ospedaliero ASST GRANDE OSPEDALE Ospedale Niguarda PIAZZA OSPEDALE MAGGIORE, 3 - 20100 - MILANO METROPOLITANO NIGUARDA Ca' Granda PO di Cernusco sul Naviglio - Vaprio VIA UBOLDO, 1 - 20063 - CERNUSCO SUL NAVIGLIO (MI) d'Adda (STAB DI CERNUSCO) Presidio Ospedaliero di Melzo-Gorgonzola VIA VOLONTARI DEL SANGUE, 5 - 20066 - MELZO (MI) (STAB DI MELZO) ASST MELEGNANO E DELLA Presidio Ospedaliero MARTESANA di Melzo-Gorgonzola VIA BELLINI - 20064 - GORGONZOLA (MI) (STAB DI GORGONZOLA) PO di Vizzolo Predabissi - Cassano VIA PANDINA, 1 - 20070- VIZZOLO PREDABISSI (MI) d'Adda (STAB DI VIZZOLO) ENTE STRUTTURA INDIRIZZO P.O. "CITTA' DI ASST NORD MILANO VIALE MATTEOTTI, 83 - 20099 - SESTO SAN GIOVANNI (MI) S.GIOVANNI" STABILIMENTO ASST OVEST MILANESE OSPEDALIERO DI VIA PAPA -

Società Di Progetto Brebemi S.P.A., C.F. E P.IVA E Iscrizione Al Registro Delle Imprese Della Camera Di Commercio Di Brescia N
Società di Progetto Brebemi S.p.A., C.F. e P.IVA e iscrizione al Registro delle Imprese della Camera di Commercio di Brescia n. 02508160989, REA 455412, capitale sociale euro 130.000.000,00, Concessionaria di Concessioni Autostradali Lombarde – CAL S.p.A. con sede in Milano Via Copernico 42, capitale sociale euro 4.000.000,00, iscrizione al Registro delle Imprese di Milano n. 05645680967, REA 1837186 AVVISO DI AVVIO DEL PROCEDIMENTO DI DICHIARAZIONE DI PUBBLICA UTILITÀ AI SENSI DEGLI ARTT. 169, COMMA 6 E 166, COMMA 2, D.LGS. 12 APRILE 2006 N. 163. INTEGRAZIONI ED ADEGUAMENTI AL PROGETTO DEFINITIVO DEL COLLEGAMENTO AUTOSTRADALE TRA LE CITTÀ DI BRESCIA E MILANO (CUP E31B05000390007) Infrastruttura strategica di preminente interesse nazionale ai sensi della L. 21 dicembre 2001 n 443 – Legge Obiettivo PREMESSO CHE -laSocietàdiProgettoBrebemiS.p.A.(d’orainavanti“Brebemi”)èConcessionariaperlaprogettazione,costruzioneegestionedelCollegamentoAutostradaletraleCittàdiBresciaeMilano,inforzadellaConvenzioneUnicadiConcessione sottoscrittaconlaConcedenteCALS.p.A.indata1agosto2007; -ilCollegamentoAutostradale,rientrantenell’ambitodel1°ProgrammadelleInfrastruttureStrategichedipreminenteinteressenazionaledicuiallaDeliberazionedelCIPEn.121del21dicembre2001,èlocalizzatonellaRegioneLombardia, segnatamentenelleProvincediBrescia,Bergamo,Milano,CremonaeLodi; -ilProgettoPreliminaredell’interventosopracitatoèstatoapprovatodalCIPEconDeliberan.93del29luglio2005,aisensieperglieffettidell’articolo3delD.Lgs.190/2002,nonchéaisensideld.P.R.n.327/2001,s.m.i,ancheaifini -
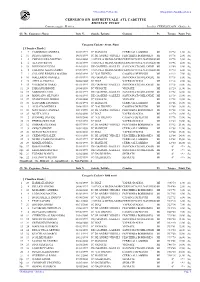
CERNUSCO S/N DISTRETTUALE ATL CADETTI/E RISULTATI FINALI Cronometraggio : Elettrico Località :CERNUSCO S/N - Giudice A
5712-6502-7345-8188 Olimpyawin Modulo atletica CERNUSCO S/N DISTRETTUALE ATL CADETTI/E RISULTATI FINALI Cronometraggio : Elettrico Località :CERNUSCO S/N - Giudice A.: Cl. Nr. Cognome - Nome Data N. Scuola / Istituto Comune Pv Tempo Punti Par. Categoria Cadetti - 80 mt. Piani [ Classifica Finale ] 1 7 CAMBRIANI ANDREA 08/07/1999 IC MANZONI CERRO AL LAMBRO MI 10"72 1,00 Sq. 2 11 DIANA SIMONE 14/01/1999 IC DE ANDRE'-VIRGILI PESCHIERA BORROMEO MI 10"76 2,00 Sq. 3 4 CERVELLERA MATTEO 30/01/2000 1 SCUOLA MEDIA MOROCERNUSCO SUL NAVIGLIO MI 10"78 3,00 Sq. 4 6 ALFANO KEVIN 13/02/1999 1 SCUOLA MEDIA MOROCERNUSCO SUL NAVIGLIO MI 10"96 4,00 Sq. 5 18 DINUNNO FULVIO 01/01/1999 DE GASPERI - GALILEI SAN DONATO MILANESE MI 11"00 5,00 Sq. 6 5 FARAONI ALESSANDRO 07/09/1999 1 SCUOLA MEDIA MOROCERNUSCO SUL NAVIGLIO MI 11"10 6,00 Sq. 7 1 CALANNI RINDINA MAURO 09/05/1999 IC VAI TRENTO CASSINA DE'PECCHI MI 11"12 7,00 Sq. 8 16 MALLARDO DANIELE 01/01/1999 DE GASPERI - GALILEI SAN DONATO MILANESE MI 11"18 8,00 Sq. 9 23 AIELLO ANGELO 04/08/2000 IC DIAZ VAPRIO D'ADDA MI 11"18 9,00 Sq. 9 15 VALBONESI DARIO 01/01/1999 DE GASPERI - GALILEI SAN DONATO MILANESE MI 11"18 10,00 Sq. 11 28 PARSANI SIMONE 29/04/1999 IC VIGNATE VIGNATE MI 11"20 11,00 Sq. 12 19 SARDONE LUIGI 01/01/1999 DE GASPERI - GALILEI SAN DONATO MILANESE MI 11"32 12,00 Sq.