Phylogeny of Green Plants Embryophytes (Land Plants) “Green
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 1-1 Introduction
Glime, J. M. 2017. Introduction. Chapt. 1. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook sponsored 1-1-1 by Michigan Technological University and the International Association of Bryologists. Last updated 25 April 2021 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 1-1 INTRODUCTION TABLE OF CONTENTS Thinking on a New Scale .................................................................................................................................... 1-1-2 Adaptations to Land ............................................................................................................................................ 1-1-3 Minimum Size..................................................................................................................................................... 1-1-5 Do Bryophytes Lack Diversity?.......................................................................................................................... 1-1-6 The "Moss".......................................................................................................................................................... 1-1-7 What's in a Name?............................................................................................................................................... 1-1-8 Phyla/Divisions............................................................................................................................................ 1-1-8 Role of Bryology................................................................................................................................................ -

Gymnosperms the MESOZOIC: ERA of GYMNOSPERM DOMINANCE
Chapter 24 Gymnosperms THE MESOZOIC: ERA OF GYMNOSPERM DOMINANCE THE VASCULAR SYSTEM OF GYMNOSPERMS CYCADS GINKGO CONIFERS Pinaceae Include the Pines, Firs, and Spruces Cupressaceae Include the Junipers, Cypresses, and Redwoods Taxaceae Include the Yews, but Plum Yews Belong to Cephalotaxaceae Podocarpaceae and Araucariaceae Are Largely Southern Hemisphere Conifers THE LIFE CYCLE OF PINUS, A REPRESENTATIVE GYMNOSPERM Pollen and Ovules Are Produced in Different Kinds of Structures Pollination Replaces the Need for Free Water Fertilization Leads to Seed Formation GNETOPHYTES GYMNOSPERMS: SEEDS, POLLEN, AND WOOD THE ECOLOGICAL AND ECONOMIC IMPORTANCE OF GYMNOSPERMS The Origin of Seeds, Pollen, and Wood Seeds and Pollen Are Key Reproductive SUMMARY Innovations for Life on Land Seed Plants Have Distinctive Vegetative PLANTS, PEOPLE, AND THE Features ENVIRONMENT: The California Coast Relationships among Gymnosperms Redwood Forest 1 KEY CONCEPTS 1. The evolution of seeds, pollen, and wood freed plants from the need for water during reproduction, allowed for more effective dispersal of sperm, increased parental investment in the next generation and allowed for greater size and strength. 2. Seed plants originated in the Devonian period from a group called the progymnosperms, which possessed wood and heterospory, but reproduced by releasing spores. Currently, five lineages of seed plants survive--the flowering plants plus four groups of gymnosperms: cycads, Ginkgo, conifers, and gnetophytes. Conifers are the best known and most economically important group, including pines, firs, spruces, hemlocks, redwoods, cedars, cypress, yews, and several Southern Hemisphere genera. 3. The pine life cycle is heterosporous. Pollen strobili are small and seasonal. Each sporophyll has two microsporangia, in which microspores are formed and divide into immature male gametophytes while still retained in the microsporangia. -

California's Native Ferns
CALIFORNIA’S NATIVE FERNS A survey of our most common ferns and fern relatives Native ferns come in many sizes and live in many habitats • Besides living in shady woodlands and forests, ferns occur in ponds, by streams, in vernal pools, in rock outcrops, and even in desert mountains • Ferns are identified by producing fiddleheads, the new coiled up fronds, in spring, and • Spring from underground stems called rhizomes, and • Produce spores on the backside of fronds in spore sacs, arranged in clusters called sori (singular sorus) Although ferns belong to families just like other plants, the families are often difficult to identify • Families include the brake-fern family (Pteridaceae), the polypody family (Polypodiaceae), the wood fern family (Dryopteridaceae), the blechnum fern family (Blechnaceae), and several others • We’ll study ferns according to their habitat, starting with species that live in shaded places, then moving on to rock ferns, and finally water ferns Ferns from moist shade such as redwood forests are sometimes evergreen, but also often winter dormant. Here you see the evergreen sword fern Polystichum munitum Note that sword fern has once-divided fronds. Other features include swordlike pinnae and round sori Sword fern forms a handsome coarse ground cover under redwoods and other coastal conifers A sword fern relative, Dudley’s shield fern (Polystichum dudleyi) differs by having twice-divided pinnae. Details of the sori are similar to sword fern Deer fern, Blechnum spicant, is a smaller fern than sword fern, living in constantly moist habitats Deer fern is identified by having separate and different looking sterile fronds and fertile fronds as seen in the previous image. -

The Chloroplast Rpl23 Gene Cluster of Spirogyra Maxima (Charophyceae) Shares Many Similarities with the Angiosperm Rpl23 Operon
Algae Volume 17(1): 59-68, 2002 The Chloroplast rpl23 Gene Cluster of Spirogyra maxima (Charophyceae) Shares Many Similarities with the Angiosperm rpl23 Operon Jungho Lee* and James R. Manhart Department of Biology, Texas A&M University, College Station, TX, 77843-3258, U.S.A. A phylogenetic affinity between charophytes and embryophytes (land plants) has been explained by a few chloro- plast genomic characters including gene and intron (Manhart and Palmer 1990; Baldauf et al. 1990; Lew and Manhart 1993). Here we show that a charophyte, Spirogyra maxima, has the largest operon of angiosperm chloroplast genomes, rpl23 operon (trnI-rpl23-rpl2-rps19-rpl22-rps3-rpl16-rpl14-rps8-infA-rpl36-rps11-rpoA) containing both embryophyte introns, rpl16.i and rpl2.i. The rpl23 gene cluster of Spirogyra contains a distinct eubacterial promoter sequence upstream of rpl23, which is the first gene of the green algal rpl23 gene cluster. This sequence is completely absent in angiosperms but is present in non-flowering plants. The results imply that, in the rpl23 gene cluster, early charophytes had at least two promoters, one upstream of trnI and another upstream of rpl23, which partially or completely lost its function in land plants. A comparison of gene clusters of prokaryotes, algal chloroplast DNAs and land plant cpDNAs indicated a loss of numerous genes in chlorophyll a+b eukaryotes. A phylogenetic analysis using presence/absence of genes and introns as characters produced trees with a strongly supported clade contain- ing chlorophyll a+b eukaryotes. Spirogyra and embryophytes formed a clade characterized by the loss of rpl5 and rps9 and the gain of trnI (CAU) and introns in rpl2 and rpl16. -

Xylans of Red and Green Algae: What Is Known About Their Structures and How They Are Synthesised?
polymers Review Xylans of Red and Green Algae: What Is Known about Their Structures and How They Are Synthesised? Yves S.Y. Hsieh 1,* and Philip J. Harris 2,* 1 Division of Glycoscience, Department of Chemistry, School of Engineering Sciences in Chemistry, Biotechnology and Health, Royal Institute of Technology (KTH), AlbaNova University Centre, SE-106 91 Stockholm, Sweden 2 School of Biological Science, The University of Auckland, Private Bag 92019, Auckland, New Zealand * Correspondence: [email protected] (Y.S.Y.H.); [email protected] (P.J.H.); Tel.: +46-8-790-9937 (Y.S.Y.H.); +64-9-923-8366 (P.J.H.) Received: 30 January 2019; Accepted: 17 February 2019; Published: 18 February 2019 Abstract: Xylans with a variety of structures have been characterised in green algae, including chlorophytes (Chlorophyta) and charophytes (in the Streptophyta), and red algae (Rhodophyta). Substituted 1,4-β-D-xylans, similar to those in land plants (embryophytes), occur in the cell wall matrix of advanced orders of charophyte green algae. Small proportions of 1,4-β-D-xylans have also been found in the cell walls of some chlorophyte green algae and red algae but have not been well characterised. 1,3-β-D-Xylans occur as triple helices in microfibrils in the cell walls of chlorophyte algae in the order Bryopsidales and of red algae in the order Bangiales. 1,3;1,4-β-D-Xylans occur in the cell wall matrix of red algae in the orders Palmariales and Nemaliales. In the angiosperm Arabidopsis thaliana, the gene IRX10 encodes a xylan 1,4-β-D-xylosyltranferase (xylan synthase), and, when heterologously expressed, this protein catalysed the production of the backbone of 1,4-β-D-xylans. -

Algae & Marine Plants of Point Reyes
Algae & Marine Plants of Point Reyes Green Algae or Chlorophyta Genus/Species Common Name Acrosiphonia coalita Green rope, Tangled weed Blidingia minima Blidingia minima var. vexata Dwarf sea hair Bryopsis corticulans Cladophora columbiana Green tuft alga Codium fragile subsp. californicum Sea staghorn Codium setchellii Smooth spongy cushion, Green spongy cushion Trentepohlia aurea Ulva californica Ulva fenestrata Sea lettuce Ulva intestinalis Sea hair, Sea lettuce, Gutweed, Grass kelp Ulva linza Ulva taeniata Urospora sp. Brown Algae or Ochrophyta Genus/Species Common Name Alaria marginata Ribbon kelp, Winged kelp Analipus japonicus Fir branch seaweed, Sea fir Coilodesme californica Dactylosiphon bullosus Desmarestia herbacea Desmarestia latifrons Egregia menziesii Feather boa Fucus distichus Bladderwrack, Rockweed Haplogloia andersonii Anderson's gooey brown Laminaria setchellii Southern stiff-stiped kelp Laminaria sinclairii Leathesia marina Sea cauliflower Melanosiphon intestinalis Twisted sea tubes Nereocystis luetkeana Bull kelp, Bullwhip kelp, Bladder wrack, Edible kelp, Ribbon kelp Pelvetiopsis limitata Petalonia fascia False kelp Petrospongium rugosum Phaeostrophion irregulare Sand-scoured false kelp Pterygophora californica Woody-stemmed kelp, Stalked kelp, Walking kelp Ralfsia sp. Silvetia compressa Rockweed Stephanocystis osmundacea Page 1 of 4 Red Algae or Rhodophyta Genus/Species Common Name Ahnfeltia fastigiata Bushy Ahnfelt's seaweed Ahnfeltiopsis linearis Anisocladella pacifica Bangia sp. Bossiella dichotoma Bossiella -

Anthocerotophyta
Glime, J. M. 2017. Anthocerotophyta. Chapt. 2-8. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook 2-8-1 sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 5 June 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-8 ANTHOCEROTOPHYTA TABLE OF CONTENTS Anthocerotophyta ......................................................................................................................................... 2-8-2 Summary .................................................................................................................................................... 2-8-10 Acknowledgments ...................................................................................................................................... 2-8-10 Literature Cited .......................................................................................................................................... 2-8-10 2-8-2 Chapter 2-8: Anthocerotophyta CHAPTER 2-8 ANTHOCEROTOPHYTA Figure 1. Notothylas orbicularis thallus with involucres. Photo by Michael Lüth, with permission. Anthocerotophyta These plants, once placed among the bryophytes in the families. The second class is Leiosporocerotopsida, a Anthocerotae, now generally placed in the phylum class with one order, one family, and one genus. The genus Anthocerotophyta (hornworts, Figure 1), seem more Leiosporoceros differs from members of the class distantly related, and genetic evidence may even present -

Anders Langangen Charophytes (Charales) from Crete (Greece) Collected in 2010
Fl. Medit. 22: 25-32 doi: 10.7320/FlMedit22.025 Version of Record published online on 28 December 2012 Anders Langangen Charophytes (Charales) from Crete (Greece) collected in 2010 Abstract Langangen, A.: Charophytes (Charales) from Crete (Greece) collected in 2010. — Fl. Medit. 22: 25-32. 2012. — ISSN: 1120-4052 printed, 2240-4538 online. In this article charophytes are reported from the island of Crete, the largest island in Greece. On 9 visited localities, charophytes have been found in six. All localities, except one (loc. 6) are freshwater. Totally six different species were found: Chara aspera, C. connivens, C. corfuen- sis, C. vulgaris, Nitella hyalina and N. tenuissima. The most interesting locality is Lake Kournas which is an eutrophic Chara-lake with rich vegetation of four species: Chara cor- fuensis, C. aspera and the two species of Nitella. Key words: Crete, Greece, Chara aspera, C. connivens, C. corfuensis, C. vulgaris, Nitella hyali- na, N. tenuissima. Introduction The island of Crete is the largest island in Greece and is situated in the southernmost part of the country. I visited several water bodies, lakes, reservoirs and seasonally wet meadows. The localities are listed in Table 1, and of nine, charophytes were found in six. Charophytes have earlier been reported from Crete in several works e.g. Corillion (1957), Koumpli-Sovantzi (1997), Bergmeister & Abrahamczyk (2008). Materials and methods This work is based on material collected in Crete (Greece) in the given localities in 2010 (Fig. 1). The specific conductivity of the water was measured with a Milwaukee, SM 301 EC meter, range 0-1990 µm/cm. -

Information and Care Instructions Mother Fern
Information and Care Instructions Mother Fern Quick Reference Botanical Name - Asplenium bulbiferum Detailed Care Exposure - Shade to bright, indirect Your Mother Fern was grown in a plastic pot. Depending on the item, it may then have been Indoor Placement - Bright location but not in direct afternoon sun transplanted into a decorative pot before sale or simply “dropped” into a container while still in the USDA Hardiness - Zone 10a to 11 plastic pot. Inside Temperature - 50-70˚F WATERING Min Outside Temperature - 30˚F 1. Let the top of the soil dry out slightly before Plant Type - Evergreen Fern watering; check frequently, especially if kept in a hot, dry spot. Mother Ferns like to be kept evenly moist, Watering - Allow soil to dry out slightly before watering. Do not allow but not soggy. the pot to sit in standing water for more than a few minutes 2. When watering, use the recommended amount of water for your pot size (See Quick Reference Guide) Water Amount Used - 4” Pot = 1/3 cup of water poured directly on the soil. In order not to damage 6 1/2” Pot = 1 1/4 cups of water your furniture, countertop or floor, place your Mother Fertilizing - Fertilize monthly Fern in a saucer, bowl or sink when watering. Allow the water to drain for 5 minutes. Do not allow the soil to sit in water for any more than 5 minutes or damage to the roots may occur. Avoid continuous use of softened water as the sodium in it can build up to damaging levels in the soil. -
Ferns of the National Forests in Alaska
Ferns of the National Forests in Alaska United States Forest Service R10-RG-182 Department of Alaska Region June 2010 Agriculture Ferns abound in Alaska’s two national forests, the Chugach and the Tongass, which are situated on the southcentral and southeastern coast respectively. These forests contain myriad habitats where ferns thrive. Most showy are the ferns occupying the forest floor of temperate rainforest habitats. However, ferns grow in nearly all non-forested habitats such as beach meadows, wet meadows, alpine meadows, high alpine, and talus slopes. The cool, wet climate highly influenced by the Pacific Ocean creates ideal growing conditions for ferns. In the past, ferns had been loosely grouped with other spore-bearing vascular plants, often called “fern allies.” Recent genetic studies reveal surprises about the relationships among ferns and fern allies. First, ferns appear to be closely related to horsetails; in fact these plants are now grouped as ferns. Second, plants commonly called fern allies (club-mosses, spike-mosses and quillworts) are not at all related to the ferns. General relationships among members of the plant kingdom are shown in the diagram below. Ferns & Horsetails Flowering Plants Conifers Club-mosses, Spike-mosses & Quillworts Mosses & Liverworts Thirty of the fifty-four ferns and horsetails known to grow in Alaska’s national forests are described and pictured in this brochure. They are arranged in the same order as listed in the fern checklist presented on pages 26 and 27. 2 Midrib Blade Pinnule(s) Frond (leaf) Pinna Petiole (leaf stalk) Parts of a fern frond, northern wood fern (p. -
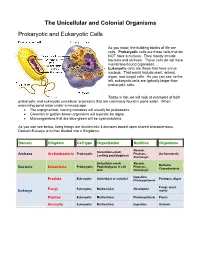
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

Coral Reef Algae
Coral Reef Algae Peggy Fong and Valerie J. Paul Abstract Benthic macroalgae, or “seaweeds,” are key mem- 1 Importance of Coral Reef Algae bers of coral reef communities that provide vital ecological functions such as stabilization of reef structure, production Coral reefs are one of the most diverse and productive eco- of tropical sands, nutrient retention and recycling, primary systems on the planet, forming heterogeneous habitats that production, and trophic support. Macroalgae of an astonish- serve as important sources of primary production within ing range of diversity, abundance, and morphological form provide these equally diverse ecological functions. Marine tropical marine environments (Odum and Odum 1955; macroalgae are a functional rather than phylogenetic group Connell 1978). Coral reefs are located along the coastlines of comprised of members from two Kingdoms and at least over 100 countries and provide a variety of ecosystem goods four major Phyla. Structurally, coral reef macroalgae range and services. Reefs serve as a major food source for many from simple chains of prokaryotic cells to upright vine-like developing nations, provide barriers to high wave action that rockweeds with complex internal structures analogous to buffer coastlines and beaches from erosion, and supply an vascular plants. There is abundant evidence that the his- important revenue base for local economies through fishing torical state of coral reef algal communities was dominance and recreational activities (Odgen 1997). by encrusting and turf-forming macroalgae, yet over the Benthic algae are key members of coral reef communities last few decades upright and more fleshy macroalgae have (Fig. 1) that provide vital ecological functions such as stabili- proliferated across all areas and zones of reefs with increas- zation of reef structure, production of tropical sands, nutrient ing frequency and abundance.