Plovers, Lapwings and Dotterels Subfamily CHARADRIINAE Leach: Plovers and Dotterels Charadriadae Leach, 1820: Eleventh Room
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meinmahla Kyun Wildlife Sanctuary Myanmar
Meinmahla Kyun Wildlife Sanctuary Myanmar EAAF NETWORK SITE CODE FOR OFFICE USE ONLY: E A A F 1 4 0 Site Information Sheet on East Asian-Australasian Flyway Network Sites (SIS) – 2017 version Available for download from http://www.eaaflyway.net/about/the-flyway/flyway-site-network/ 1 of 22 Information Sheet on EAA Flyway Network Sites | Meinmahla Kyun Wildlife Sanctuary [EAAF140] Categories approved by Second Meeting of the Partners of the East Asian-Australasian Flyway Partnership in Beijing, China 13-14 November 2007 - Report (Minutes) Agenda Item 3.13 Notes for compilers: 1. The management body intending to nominate a site for inclusion in the East Asian - Australasian Flyway Site Network is requested to complete a Site Information Sheet. The Site Information Sheet will provide the basic information of the site and detail how the site meets the criteria for inclusion in the Flyway Site Network. When there is a new nomination or an SIS update, the following sections with an asterisk (*), from Questions 1-14 and Question 30, must be filled or updated at least so that it can justify the international importance of the habitat for migratory waterbirds. 2. The Site Information Sheet is based on the Ramsar Information Sheet. If the site proposed for the Flyway Site Network is an existing Ramsar site then the documentation process can be simplified. 3. Once completed, the Site Information Sheet (and accompanying map(s)) should be submitted to the Secretariat. Compilers should provide an electronic (MS Word) copy of the Information Sheet and, where possible, digital versions (e.g. -

The Wrybill <I>Anarhynchus Frontalis</I>: a Brief Review of Status, Threats and Work in Progress
The Wrybill Anarhynchus frontalis: a brief review of status, threats and work in progress ADRIAN C. RIEGEN '1 & JOHN E. DOWDING 2 •231 ForestHill Road, Waiatarua, Auckland 8, NewZealand, e-maih riegen @xtra.co. nz; 2p.o. BOX36-274, Merivale, Christchurch 8030, New Zealand, e-maih [email protected]. nz Riegen,A.C. & Dowding, J.E. 2003. The Wrybill Anarhynchusfrontalis:a brief review of status,threats and work in progress.Wader Study Group Bull. 100: 20-24. The Wrybill is a threatenedplover endemic to New Zealandand unique in havinga bill curvedto the right.It is specializedfor breedingon bareshingle in thebraided riverbeds of Canterburyand Otago in the SouthIsland. After breeding,almost the entirepopulation migrates north and wintersin the harboursaround Auckland. The speciesis classifiedas Vulnerable. Based on countsof winteringflocks, the population currently appears to number4,500-5,000 individuals.However, countingproblems mean that trendsare difficult to determine. The mainthreats to theWrybill arebelieved to be predationon thebreeding grounds, degradation of breeding habitat,and floodingof nests.In a recentstudy in the MackenzieBasin, predation by introducedmammals (mainly stoats,cats and possibly ferrets) had a substantialimpact on Wrybill survivaland productivity. Prey- switchingby predatorsfollowing the introductionof rabbithaemorrhagic disease in 1997 probablyincreased predationrates on breedingwaders. A recentstudy of stoatsin the TasmanRiver showedthat 11% of stoat densexamined contained Wrybill remains.Breeding habitat is beinglost in somerivers and degraded in oth- ers,mainly by waterabstraction and flow manipulation,invasion of weeds,and human recreational use. Flood- ing causessome loss of nestsbut is alsobeneficial, keeping nesting areas weed-free. The breedingrange of the speciesappears to be contractingand fragmenting, with the bulk of the popula- tion now breedingin three large catchments. -

The First Record of Common Ringed Plover (Charadrius Hiaticula) in British Columbia
The First Record of Common Ringed Plover (Charadrius hiaticula) in British Columbia. By Rick Toochin. Submitted: April 15, 2019. Introduction and Distribution The Common Ringed Plover (Charadrius hiaticula) is a widespread Old World shorebird species that is found breeding in the Arctic and subarctic regions from Greenland, Europe, east to Siberia (O’Brien et al. 2006). In North America, this species breeds on Baffin Island, eastern Ellesmere Island (Godfrey 1986). The Common Ringed Plover winters primarily from Western Europe, the Mediterranean Basin, throughout Africa, including Madagascar, and the Middle East (Hayman et al. 1986, O’Brien et al. 2006, Brazil 2009). There are three recognized subspecies of the Common Ringed Plover (Thies et al. 2018). Distinction between the subspecies is based on moult; with features changing clinally North to South, rather than East to West, making it impossible to draw a dividing line in Northwestern Europe (del Hoyo et al. 1996, Snow and Perrins 1998). The nominate subspecies of Common Ringed Plover is (C. h. hiaticula ) which breeds from southern Scandinavia to Great Britain, and northwestern France (Wiersma et al. 2019). This subspecies winters from Great Britain, south into Africa (del Hoyo et al. 1996, Snow and Perrins 1998). The second subspecies of the Common Ringed Plover is (C. h. tundrae) which is found breeding from northern Scandinavia, and northern Russia east to the Chukotskiy Peninsula, and is a casual breeder also in the northern Bering Sea region of Alaska on St Lawrence Island (Wiersma et al. 2019). This subspecies winters in the Caspian Sea region, and from Southwest Asia, south and east to South Africa (Wiersma et al. -

Species Assessment for Mountain Plover (Charadrius Montanus)
SPECIES ASSESSMENT FOR MOUNTAIN PLOVER (CHARADRIUS MONTANUS ) IN WYOMING prepared by 1 2 HAMILTON SMITH AND DOUGLAS A. KEINATH 1 Wyoming Natural Diversity Database, University of Wyoming, 1000 E. University Ave, Dept. 3381, Laramie, Wyoming 82071; 307-766-3023 2 Zoology Program Manager, Wyoming Natural Diversity Database, University of Wyoming, 1000 E. University Ave, Dept. 3381, Laramie, Wyoming 82071; 307-766-3013; [email protected] drawing by Summers Scholl prepared for United States Department of the Interior Bureau of Land Management Wyoming State Office Cheyenne, Wyoming November 2004 Smith and Keinath – Charadrius montanus November 2004 Table of Contents INTRODUCTION ................................................................................................................................. 3 NATURAL HISTORY ........................................................................................................................... 4 Morphological Description ...................................................................................................... 4 Taxonomy and Distribution ..................................................................................................... 5 Habitat Requirements............................................................................................................. 6 General ............................................................................................................................................6 Breeding ..........................................................................................................................................7 -

Helminth Parasite Communities in Four Species of Shorebirds (Charadriidae) on King Island, Tasmania
Papers and Proceedings of the Royal Society of Tasmania, Volume 132, 1998 49 HELMINTH PARASITE COMMUNITIES IN FOUR SPECIES OF SHOREBIRDS (CHARADRIIDAE) ON KING ISLAND, TASMANIA by Albert G. Canaris and John M. Kinsella (with six tables and three text-figures) CANARIS, A.G. & KINSELLA, J.M., 1998 (31 :xii): Helminth parasite communities in four species of shorebirds (Charadriidae) on King Island, Tasmania. Pap. Proc. R. Soc. Tasm., 132: 49-57. https://doi.org/10.26749/rstpp.132.49 ISSN 0080-4703. PO Box 717, Hamilton, Montana, USA 59840 (formerly Department of Biological Sciences, University of Texas at El Paso) (AGC); and Department of lnfectious Diseases, College of Veterinary Medicine, University of Florida, Gainesville, Florida, USA 32611 QMK). Helminth community composition and structure were examined among two resident shorebird species, red-capped plover, Charadrius ruficapillus (N = 20), and masked lapwing, Vanellusmiles (N = 5), and two migrants, ruddy turnstone, Arenaria interpres (N = 20), and curlew sandpiper, Calidrisfe rruginea (N = 5), on King Island, Tasmania in March-April 1993, prior to northward migration to the nesting grounds. The total number of species of helminths recovered was 28 and life cycles of at least 19 of these were occurring on the island. Twenty-fivespecies were categorised as generalists and three were undetermined. One to three species of helminths were dominant in each host species. Eight species, to various degrees, were common among the four species of host. Most sharing occurred in the mucosa! trematode guild. Similarities between resident Charadrius ruficapillus and migrant A. interpres was 32.7%, while the mean number of species and mean number of helminths were significantlyhigher in A. -
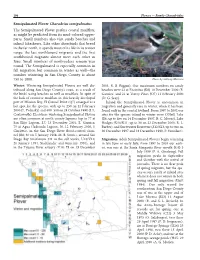
Semipalmated Plover Charadrius Semipalmatus the Semipalmated Plover Prefers Coastal Mudflats, As Might Be Predicted from Its Mud-Colored Upper- Parts
196 Plovers — Family Charadriidae Semipalmated Plover Charadrius semipalmatus The Semipalmated Plover prefers coastal mudflats, as might be predicted from its mud-colored upper- parts. Small numbers also visit sandy beaches and inland lakeshores. Like other shorebirds that breed in the far north, it spends most of its life in its winter range: the last northbound migrants and the first southbound migrants almost meet each other in June. Small numbers of nonbreeders remain year round. The Semipalmated is especially common in fall migration but common in winter as well—the number wintering in San Diego County is about 750 to 1000. Photo by Anthony Mercieca Winter: Wintering Semipalmated Plovers are well dis- 2001, R. B. Riggan). Our maximum numbers on sandy tributed along San Diego County’s coast, as a result of beaches were 23 at Encinitas (K6) 10 December 2000 (E. the birds’ using beaches as well as mudflats. In spite of Garnica) and 21 at Torrey Pines (O7) 11 February 2000 the lack of extensive mudflats in this heavily developed (D. G. Seay). part of Mission Bay, El Carmel Point (Q7) emerged as a Inland the Semipalmated Plover is uncommon in hot spot for the species, with up to 250 on 12 February migration and generally rare in winter, when it has been 2000 (L. Polinsky) and 400–500 on 24 October 1998 (J. L. found only in the coastal lowland. From 1997 to 2002 our Coatsworth). Elsewhere wintering Semipalmated Plovers sites for the species inland in winter were O’Neill Lake are often common at north county lagoons (up to 77 at (E6; up to five on 14 December 1997, B. -

Plover, Snowy
Plovers — Family Charadriidae 193 Snowy Plover Charadrius alexandrinus Nesting on beaches, dunes, and salt flats, the Snowy Plover is among San Diego County’s scarcest and most threatened breeding birds. In 1993 the U.S. Fish and Wildlife Service listed it as threatened along the entire Pacific coast. Thorough surveys from 1995 to 1998 put the county’s breeding population between 240 and 325 individuals, most concentrated in two areas, Camp Pendleton and the Silver Strand (Powell et al. 2002). Surveys from 1978 to 1998 suggest the decline of this once common bird has continued. When not breeding the Snowy Plover is more wide- spread along the county’s coast but it is not much Photo by Anthony Mercieca more numerous, in spite of considerable migration. Human disturbance of the remaining habitat and a high level of predation mean that intensive manage- single breeding season, some individuals shift from site to site, although most remain at one site. ment is needed to sustain the population. These variables considered, about half the population Breeding distribution: Powell et al. (2002) surveyed San breeds in Camp Pendleton, with six to eight nests per year Diego County’s coastline intensively from 1994 to 1998 at the mouths of Aliso and French creeks (F4) and 67 to and provided an exhaustive view of the Snowy Plover’s 88 at the Santa Margarita River mouth (G4). The high distribution. Their detailed table enumerated nests rather count of individual plovers was 120 at the latter site 11 than birds per site; because of multiple clutches per year, June 1997 (B. -

Breeding Biology of the Mountain Plover
BREEDING BIOLOGY OF THE MOUNTAIN PLOVER WALTER D. GRAUL The Mountain Plover (Charadrius montanus) is an endemic species of western North America, breeding on the shortgrass prairie mainly east of the Rocky Mountains and wintering in similar habitat from California and Texas to northern Mexico. Apart from a few anecdotal reports, detailed informa- tion on the breeding biology of the species comes from a single study (Laun 1957). In 1969, I began a study of the Mountain Plover on its Colorado breeding grounds. This paper describes various aspects of the breeding biology of the species and discusses their adaptive significance. Other aspects of the behavior of the Mountain Plover have been published elsewhere (Graul1973a, 1973b,l974). STUDY AREA AND METHODS I studied these plovers on two areas in northern Weld Co., northeastern Colorado. The major area consisted of 16 km ’ just southwest of Keota. The second area was on the International Biological Programs’ Pawnee Site, approximately 64 km northwest of Keota. This general area is part of the high shortgrass prairie (elevation about 1470 m) and consists of gently rolling hills with extensive flats and intermittent streams. Most observations were confined to areas covered predominantly by blue grama grass (Bout&ma gracilis) and/or buffalo grass (Buchloe dactyloides), since I observed that Mountain Plovers prefer such areas. In these areas other common plants include western wheat grass (Agropyron smithii), fourwing saltbush (Atriplex canescens) , and prickly pear cactus (Opuntia polycantha) . The climate of the area during the breeding season is hot and dry. Average yearly precipitation ranges from 30 to 38 cm, although precipitation is unevenly distributed on a yearly, seasonal, and area-to-area basis (Badaracco 1971). -

SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does Not Include Alcidae
SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does not include Alcidae CREATED BY AZA CHARADRIIFORMES TAXON ADVISORY GROUP IN ASSOCIATION WITH AZA ANIMAL WELFARE COMMITTEE Shorebirds (Charadriiformes) Care Manual Shorebirds (Charadriiformes) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Charadriiformes Taxon Advisory Group. (2014). Shorebirds (Charadriiformes) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: October 2013 Authors and Significant Contributors: Aimee Greenebaum: AZA Charadriiformes TAG Vice Chair, Monterey Bay Aquarium, USA Alex Waier: Milwaukee County Zoo, USA Carol Hendrickson: Birmingham Zoo, USA Cindy Pinger: AZA Charadriiformes TAG Chair, Birmingham Zoo, USA CJ McCarty: Oregon Coast Aquarium, USA Heidi Cline: Alaska SeaLife Center, USA Jamie Ries: Central Park Zoo, USA Joe Barkowski: Sedgwick County Zoo, USA Kim Wanders: Monterey Bay Aquarium, USA Mary Carlson: Charadriiformes Program Advisor, Seattle Aquarium, USA Sara Perry: Seattle Aquarium, USA Sara Crook-Martin: Buttonwood Park Zoo, USA Shana R. Lavin, Ph.D.,Wildlife Nutrition Fellow University of Florida, Dept. of Animal Sciences , Walt Disney World Animal Programs Dr. Stephanie McCain: AZA Charadriiformes TAG Veterinarian Advisor, DVM, Birmingham Zoo, USA Phil King: Assiniboine Park Zoo, Canada Reviewers: Dr. Mike Murray (Monterey Bay Aquarium, USA) John C. Anderson (Seattle Aquarium volunteer) Kristina Neuman (Point Blue Conservation Science) Sarah Saunders (Conservation Biology Graduate Program,University of Minnesota) AZA Staff Editors: Maya Seaman, MS, Animal Care Manual Editing Consultant Candice Dorsey, PhD, Director of Animal Programs Debborah Luke, PhD, Vice President, Conservation & Science Cover Photo Credits: Jeff Pribble Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

Migratory Shorebird Guild
Migratory Shorebird Guild Piping Plover Charadrius melodus Sanderling Calidris alba Semipalmated Plover Charadrius semipalmatus Red Knot Calidris canutus Black-bellied Plover Pluvialis squatarola Marbled Godwit Limosa fedoa American Golden Plover Pluvialis dominica Buff-breasted Sandpiper Tryngites subruficollis Wimbrel Numenius phaeopus White-rumped Sandpiper Calidris fuscicollis Long-billed Curlew Numenius americanus Pectoral Sandpiper Calidris melanotos Greater Yellowlegs Tringa melanoleuca Purple Sandpiper Calidris maritima Lesser Yellowlegs Tringa flavipes Stilt Sandpiper Calidris himantopus Solitary Sandpiper Tringa solitaria Wilson’s Snipe Gallinago gallinago delicata Spotted Sandpiper Actitis macularia American Avocet Recurvirostra Americana Upland Sandpiper Bartramia longicauda Least Sandpiper Calidris minutilla Semipalmated Sandpiper Calidris pusilla Short-billed Dowitcher Limnodromus griseus Western Sandpiper Calidris mauri Long-billed Dowitcher Limnodromus scolopaceus Dunlin Calidris alpina Contributors: Felicia Sanders and Thomas M. Murphy DESCRIPTION Photograph by SC DNR Taxonomy and Basic Description The migratory shorebird guild is composed of birds in the Charadrii suborder. Migrants in South Carolina represent three families: Scolopacidae (sandpipers), Charadriidae (plovers) and Recurvirostridae (avocets). Sandpipers are the most diverse family of shorebirds. Their tactile foraging strategy encompasses probing in soft mud or sand for invertebrates. Plovers are medium size birds, with relatively short, thick bills and employ a distinctive foraging strategy. They stand, looking for prey and then run to feed on detected invertebrates. Avocets are large shorebirds with long recurved bills and partial webbing between the toes. They feed employing both tactile and visual methods. Shorebirds are characterized by long legs for wading and wings designed for quick flight and transcontinental migrations. Migrations can span continents; for example, red knots migrate from the Canadian arctic to the southern tip of South America. -

Biometrics and Breeding Phenology of Terek Sandpipers in the Pripyat’ Valley, S Belarus
54 Wader Study Group Bulletin Biometrics and breeding phenology of Terek Sandpipers in the Pripyat’ Valley, S Belarus NATALIA KARLIONOVA1, MAGDALENA REMISIEWICZ2, PAVEL PINCHUK1 1Institute of Zoology, Belarussian National Academy of Sciences, Academichnaya Str. 27, 220072 Minsk, Belarus. [email protected] 2Avian Ecophysiology Unit, Dept of Vertebrate Ecology and Zoology, Univ. of Gdansk, al. Legionów 9, 80-441 Gdansk, Poland. [email protected] Karlionova, N., Remisiewicz, M. & Pinchuk, P. 2006. Biometrics and breeding phenology of Terek Sandpipers in the Pripyat’ Valley, S Belarus. Wader Study Group Bull. 110: 54–58. Keywords: Terek Sandpiper, Xenus cinereus, biometrics, breeding phenology, Pripyat’ Valley, Belarus We present data on the breeding phenology and biometrics of Terek Sandpipers from the isolated westernmost population in the Pripyat’ river valley, S Belarus, close to the border with Ukraine. Studies were conducted on floodplain islands between the beginning of April and mid-July during 1996–1999 and 2002–2006. Over the years, the first arrivals appeared during 10–26 April (median 14 April), first eggs were laid during 24 April to 5 May (median 30 April) and the latest egg was laid on 25 May, first chicks hatched during 19 May to 1 June (median 25 May) and the first fledged juveniles were caught on 23 June. We present biometric data for juve- niles (at the post-fledging stage) and adults. The mean wing length of juveniles, just before departure from the breeding grounds in mid June, reached 96% of that of adults. Juvenile total head lengths were 91% of adult, and bill and nalospi lengths 85% of adult, but tarsus and tarsus plus toe lengths were the same as adults. -

The Effects of Management Practices on Grassland Birds—Mountain Plover (Charadrius Montanus)
The Effects of Management Practices on Grassland Birds— Mountain Plover (Charadrius montanus) Chapter E of The Effects of Management Practices on Grassland Birds Professional Paper 1842–E U.S. Department of the Interior U.S. Geological Survey Cover. Mountain Plover. Photograph by Ron Knight, used with permission. Background photograph: Northern mixed-grass prairie in North Dakota, by Rick Bohn, used with permission. The Effects of Management Practices on Grassland Birds—Mountain Plover (Charadrius montanus) By Jill A. Shaffer,1 Lawrence D. Igl,1 Douglas H. Johnson,1 Marriah L. Sondreal,1 Christopher M. Goldade,1,2 Melvin P. Nenneman,1,3 Travis L. Wooten,1,4 and Betty R. Euliss1 Chapter E of The Effects of Management Practices on Grassland Birds Edited by Douglas H. Johnson,1 Lawrence D. Igl,1 Jill A. Shaffer,1 and John P. DeLong1,5 1U.S. Geological Survey. 2South Dakota Game, Fish and Parks (current). 3U.S. Fish and Wildlife Service (current). 4San Diego Zoo Institute for Conservation Research (current). 5University of Nebraska-Lincoln (current). Professional Paper 1842–E U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DAVID BERNHARDT, Secretary U.S. Geological Survey James F. Reilly II, Director U.S. Geological Survey, Reston, Virginia: 2019 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit https://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit https://store.usgs.gov.