Pericarditis and Myocarditis Long After SARS-Cov-2 Infection: a Cross-Sectional Descriptive Study in Health-Care Workers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -

Cardiac Involvement in COVID-19 Patients: a Contemporary Review
Review Cardiac Involvement in COVID-19 Patients: A Contemporary Review Domenico Maria Carretta 1, Aline Maria Silva 2, Donato D’Agostino 2, Skender Topi 3, Roberto Lovero 4, Ioannis Alexandros Charitos 5,*, Angelika Elzbieta Wegierska 6, Monica Montagnani 7,† and Luigi Santacroce 6,*,† 1 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Coronary Unit and Electrophysiology/Pacing Unit, Cardio-Thoracic Department, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] 2 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Cardiac Surgery, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] (A.M.S.); [email protected] (D.D.) 3 Department of Clinical Disciplines, School of Technical Medical Sciences, University of Elbasan “A. Xhuvani”, 3001 Elbasan, Albania; [email protected] 4 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Clinical Pathology Unit, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] 5 Emergency/Urgent Department, National Poisoning Center, Riuniti University Hospital of Foggia, 71122 Foggia, Italy 6 Department of Interdisciplinary Medicine, Microbiology and Virology Unit, University of Bari “Aldo Moro”, Piazza G. Cesare, 11, 70124 Bari, Italy; [email protected] 7 Department of Biomedical Sciences and Human Oncology—Section of Pharmacology, School of Medicine, University of Bari “Aldo Moro”, Policlinico University Hospital of Bari, p.zza G. Cesare 11, 70124 Bari, Italy; [email protected] * Correspondence: [email protected] (I.A.C.); [email protected] (L.S.) † These authors equally contributed as co-last authors. Citation: Carretta, D.M.; Silva, A.M.; D’Agostino, D.; Topi, S.; Lovero, R.; Charitos, I.A.; Wegierska, A.E.; Abstract: Background: The widely variable clinical manifestations of SARS-CoV2 disease (COVID-19) Montagnani, M.; Santacroce, L. -

J Wave Syndromes
Review Article http://dx.doi.org/10.4070/kcj.2016.46.5.601 Print ISSN 1738-5520 • On-line ISSN 1738-5555 Korean Circulation Journal J Wave Syndromes: History and Current Controversies Tong Liu, MD1, Jifeng Zheng, MD2, and Gan-Xin Yan, MD3,4 1Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular disease, Department of Cardiology, Tianjin Institute of Cardiology, The Second Hospital of Tianjin Medical University, Tianjin, 2Department of cardiology, The Second Hospital of Jiaxing, Jiaxing, China, 3Lankenau Institute for Medical Research and Lankenau Medical Center, Wynnewood, Pennsylvania, USA, 4The First Affiliated Hospital, Medical School of Xi'an Jiaotong University, Xi'an, China The concept of J wave syndromes was first proposed in 2004 by Yan et al for a spectrum of electrocardiographic (ECG) manifestations of prominent J waves that are associated with a potential to predispose affected individuals to ventricular fibrillation (VF). Although the concept of J wave syndromes is widely used and accepted, there has been tremendous debate over the definition of J wave, its ionic and cellular basis and arrhythmogenic mechanism. In this review article, we attempted to discuss the history from which the concept of J wave syndromes (JWS) is evolved and current controversies in JWS. (Korean Circ J 2016;46(5):601-609) KEY WORDS: Brugada syndrome; Sudden cardiac death; Ventricular fibrillation. Introduction History of J wave and J wave syndromes The concept of J wave syndromes was first proposed in 2004 The J wave is a positive deflection seen at the end of the QRS by Yan et al.1) for a spectrum of electrocardiographic (ECG) complex; it may stand as a distinct “delta” wave following the QRS, manifestations of prominent J waves that are associated with a or be partially buried inside the QRS as QRS notching or slurring. -

The Syndrome of Alternating Bradycardia and Tachycardia by D
Br Heart J: first published as 10.1136/hrt.16.2.208 on 1 April 1954. Downloaded from THE SYNDROME OF ALTERNATING BRADYCARDIA AND TACHYCARDIA BY D. S. SHORT From the National Heart Hospita. Received September 15, 1953 Among the large number of patients suffering from syncopal attacks who attended the National Heart Hospital during a four-year period, there were four in whom examination revealed sinus bradycardia alternating with prolonged phases of auricular tachycardia. These patients presented a difficult problem in treatment. Each required at least one admission to hospital and in one case the symptoms were so intractable as to necessitate six admissions in five years. Two patients had mitral valve disease, one of them with left bundle branch block. One had aortic valve sclerosis while the fourth had no evidence of heart disease. THE HEART RATE The sinus rate usually lay between 30 and 50 a minute, a rate as slow as 22 a minute being observed in one patient (Table I). Sinus arrhythmia was noted in all four patients, wandering of TABLE I http://heart.bmj.com/ RATE IN SINus RHYTHM AND IN AURICULAR TACHYCARDIA Rate in Case Age Sex Associated Rate in auricular tachycardia heart disease sinus rhythm Auricular Venliicular 1 65 M Aortic valve sclerosis 28-48 220-250 60-120 2 47 F Mitral valve disease 35-75 180-130 90-180 on September 26, 2021 by guest. Protected copyright. 3 38 F Mitral valve disease 22-43 260 50-65 4 41 F None 35-45 270 110 the pacemaker in three, and periods of sinus standstill in two (Fig. -

Pericardial Effusion
Pericardial Effusion ABOUT THE DIAGNOSIS are incurable, and treatment is designed to extend life and keep Pericardial effusion refers to an accumulation of fluid around the heart, the pet comfortable. Other underlying causes may be correctable, within the pericardium. The pericardium is a membranous sac that such as foreign bodies or coagulation disorders. surrounds the heart. When fluid accumulates slowly, the pericardium stretches and enlarges to accommodate the fluid, meaning that symp- TREATMENT toms are absent or delayed. A more rapid accumulation can cause If cardiac tamponade is present, the fluid must be drained promptly immediate symptoms, even with relatively small amounts of pericardial by a procedure called pericardiocentesis. Using local anesthetic, your fluid accumulation. The presence of fluid causes symptoms because veterinarian passes a catheter between the ribs into the pericardial the fluid compresses the heart and interferes with normal filling of the sac, and the fluid is drawn off. Alleviating the fluid accumulation that heart with blood. Less blood filling the heart means that less blood compresses the heart will rapidly stabilize a pet’s circulation and is pumped to the body with each heartbeat. Pericardial effusion can cardiovascular status in the vast majority of cases. Treatment then increase the external pressure on the heart to the point that delivery of depends upon the cause of the condition. If the underlying condition blood to the body is severely compromised, a condition called cardiac cannot be corrected, sometimes a procedure called pericardiectomy tamponade. Severe cardiac tamponade is a life-threatening condition. is performed. This is a surgery of the chest in which the pericardial Pericardial effusion is more common in older, large breed dogs. -

Pericardial Disease and Other Acquired Heart Diseases
Royal Brompton & Harefield NHS Foundation Trust Pericardial disease and other acquired heart diseases Sylvia Krupickova Exam oriented Echocardiography course, 4th November 2016 Normal Pericardium: 2 layers – fibrous - serous – visceral and parietal layer 2 pericardial sinuses – (not continuous with one another): • Transverse sinus – between in front aorta and pulmonary artery and posterior vena cava superior • Oblique sinus - posterior to the heart, with the vena cava inferior on the right side and left pulmonary veins on the left side Normal pericardium is not seen usually on normal echocardiogram, neither the pericardial fluid Acute Pericarditis: • How big is the effusion? (always measure in diastole) • Where is it? (appears first behind the LV) • Is it causing haemodynamic compromise? Small effusion – <10mm, black space posterior to the heart in parasternal short and long axis views, seen only in systole Moderate – 10-20 mm, more than 25 ml in adult, echo free space is all around the heart throughout the cardiac cycle Large – >20 mm, swinging motion of the heart in the pericardial cavity Pericardiocentesis Constrictive pericarditis Constriction of LV filling by pericardium Restriction versus Constriction: Restrictive cardiomyopathy Impaired relaxation of LV Constriction versus Restriction Both have affected left ventricular filling Constriction E´ velocity is normal as there is no impediment to relaxation of the left ventricle. Restriction E´ velocity is low (less than 5 cm/s) due to impaired filling of the ventricle (impaired relaxation) -

Case Report: Cytarabine-Induced Pericarditis and Pericardial Effusion Rino Sato, MD and Robert Park, MD
HEMATOLOGY & ONCOLOGY Case Report: Cytarabine-Induced Pericarditis and Pericardial Effusion Rino Sato, MD and Robert Park, MD INTRODUCTION for inpatient chemotherapy, and demonstrated mild global left ventricular dysfunction with ejection fraction Cytarabine (cytosine arabinoside, Ara-C) is an antime- of 40%. The cardiomyopathy was attributed to his tabolite analogue of cytidine that is used as a chemo- underlying hypertension or sleep apnea, and not therapeutic agent for the treatment of acute myelogenous coronary artery disease based on a normal coronary leukemia and lymphocytic leukemias1 . The most computed tomography (CT) angiogram. The patient common side effects of this therapy include myelosup- was started on induction therapy with high-dose pression, pancytopenia, hepatotoxicity, gastrointestinal cytarabine therapy at 3g/m2 every twelve hours without ulceration with bleeding, and pulmonary infiltrates2. an anthracycline agent such as doxorubicin. Cardio-pulmonary complications of cytarabine therapy are uncommon, but include supraventricular and On day 5 of cytarabine therapy, the patient developed ventricular arrhythmias, sinus bradycardia, and recurrent non-radiating sharp chest pain that worsened with heart failure2, 3. Occasionally, patients may develop inspiration and palpation. He had no cough or sputum pericarditis leading to pericardial tamponade, which can production. His cardiac exam revealed a tri-phasic, be fatal. We report a case of cytarabine-induced high-pitched friction rub best heard over the left lower pericarditis and pericardial effusion to increase awareness sternal border. He was normotensive, did not have pulsus about this serious side effect of cytarabine and review paradoxus, and had minimally distended jugular veins. the current literature. An electrocardiogram revealed widespread concave ST-elevation and PR-depression in the limb leads (I, II, III, CASE PRESENTATION avF) and precordial leads (V5-V6) concerning for acute pericarditis (Figure 1). -

Acute Non-Specific Pericarditis R
Postgrad Med J: first published as 10.1136/pgmj.43.502.534 on 1 August 1967. Downloaded from Postgrad. med. J. (August 1967) 43, 534-538. CURRENT SURVEY Acute non-specific pericarditis R. G. GOLD * M.B., B.S., M.RA.C.P., M.R.C.P. Senior Registrar, Cardiac Department, Brompton Hospital, London, S.W.3 Incidence neck, to either flank and frequently through to the Acute non-specific pericarditis (acute benign back. Occasionally pain is experienced on swallow- pericarditis; acute idiopathic pericarditis) has been ing (McGuire et al., 1954) and this was the pre- recognized for over 100 years (Christian, 1951). In senting symptom in one of our own patients. Mild 1942 Barnes & Burchell described fourteen cases attacks of premonitory chest pain may occur up to of the condition and since then several series of 4 weeks before the main onset of symptoms cases have been published (Krook, 1954; Scherl, (Martin, 1966). Malaise is very common, and is 1956; Swan, 1960; Martin, 1966; Logue & often severe and accompanied by listlessness and Wendkos, 1948). depression. The latter symptom is especially com- Until recently Swan's (1960) series of fourteen mon in patients suffering multiple relapses or patients was the largest collection of cases in this prolonged attacks, but is only partly related to the country. In 1966 Martin was able to collect most length of the illness and fluctuates markedly from of his nineteen cases within 1 year in a 550-bed day to day with the patient's general condition. hospital. The disease is thus by no means rare and Tachycardia occurs in almost every patient at warrants greater attention than has previously some stage of the illness. -

Pericardial Effusion in Three Cases of Anorexia Nervosa
KoreanJournalofPediatricsVol.51,No.2,2008 DOI : 10.3345/kjp.2008.51.2.209 □ Case Report □ 1) Pericardial effusion in three cases of anorexia nervosa Young Kuk Cho, M.D., Su Jin Yang, M.D.* and Jae Sook Ma, M.D. Department of Pediatrics and Psychiatry*, Chonnam National University Medical School and Research Institute of Medical Sciences, Gwangju, Korea In young adolescent girls, anorexia nervosa is a significant cause of weight loss, and hospital admis- sions among children and adolescents. Anorexia nervosa is a life-threatening disorder, with about one-third of deaths caused by cardiac complications. A high rate of pericardial effusion has been recently reported in patients with anorexia nervosa, although relatively few cases require pericardio- centesis. Here, we describe three patients with anorexia nervosa who were diagnosed with large peri- cardial effusions. To prevent cardiac tamponade, pericardiocentesis was performed in two girls. (Korean J Pediatr 2008;51:209-213) Key Words : Pericardial effusion, Anorexia nervosa, Cardiac tamponade pericardiocentesis to prevent cardiac tamponade. Introduction Case Report Anorexia nervosa is an eating disorder that is charac- terized by an intense fear of gaining weight, placing undue Case 1 emphasis on body shape, having a body weight less than 85% of the predicted weight, and amenorrhea for three con- A 14-year-old girl with anorexia nervosa was admitted secutive periods1).Itisthemaincauseofweightlossin for clinical evaluation and treatment. She began her restric- children and adolescents and accounts for numerous hospital tive eating behavior 6 months prior to this visit, which admissions.Theprevalenceisabout0.3%inyoungwomen resulted in a weight loss of 13 kg. -

Pericardial Effusion Andmitral Valve Involvement in Systemic Lupus
Ann Rheum Dis: first published as 10.1136/ard.36.4.349 on 1 August 1977. Downloaded from Annals of the Rheumatic Diseases, 1977, 36, 349-353 Pericardial effusion and mitral valve involvement in systemic lupus erythematosus Echocardiographic study URI ELKAYAM, SHMUEL WEISS, AND SHLOMO LANIADO From the Gradel Heart Department, and the Department of Rheumatology and Rehabilitation, Municipal Governmental Medical Center, Ichilov Hospital, and Sackler School ofMedicine, Tel-Aviv University, Tel-Aviv, Israel SUMMARY Echocardiography was used in 30 women and 2 men with systemic lupus erythematosus (SLE) in order to determine the incidence and severity of pericardial effusion and mitral valve involvement. 31 patients showed normal thickness of the mitral valve leaflets, only one patient showed irregular thickening of the leaflets suggesting the presence of vegetations. Mitral valve motions were normal in all patients. These results indicate that myocardial and valvular involvement in SLE is usually not severe enough to result in haemodynamic abnormalities. Pericardial effusion was found in 2 patients who were symptom free, whereas 4 of the patients with a past history suggestive of pericarditis showed no echocardiographic evidence of pericardial effusion. These copyright. suggest the transient nature of pericarditis in SLE, and the value ofechocardiography as a diagnostic tool in detecting clinically inapparent lupus pericarditis. Since Libman and Sachs (1924) first described cardiac detecting pericardial effusion (Feigenbaum, 1970; involvement in systemic lupus erythematosus (SLE), Horowitz et al., 1974) and assessing abnormalities many clinical and pathological studies have shown in movement and form of the mitral valve cusps http://ard.bmj.com/ that different heart tissues may be affected (Tauben- (Edler 1961; Zaky et al., 1968; Dillon et al., 1973). -

Case Report Chagas Cardiomyopathy Presenting As Symptomatic Bradycardia: an Underappreciated Emerging Public Health Problem in the United States
Hindawi Case Reports in Cardiology Volume 2017, Article ID 5728742, 5 pages https://doi.org/10.1155/2017/5728742 Case Report Chagas Cardiomyopathy Presenting as Symptomatic Bradycardia: An Underappreciated Emerging Public Health Problem in the United States Richard Jesse Durrance,1 Tofura Ullah,1 Zulekha Atif,1 William Frumkin,2 and Kaushik Doshi1 1 Department of Internal Medicine, Jamaica Hospital Medical Center, 8900 Van Wyck Expressway, Jamaica, NY 11418, USA 2Department of Cardiology, Jamaica Hospital Medical Center, 8900 Van Wyck Expressway, Jamaica, NY 11418, USA Correspondence should be addressed to Richard Jesse Durrance; [email protected] Received 13 February 2017; Accepted 18 July 2017; Published 16 August 2017 Academic Editor: Aiden Abidov Copyright © 2017 Richard Jesse Durrance et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Chagas cardiomyopathy (CCM) is traditionally considered a disease restricted to areas of endemicity. However, an estimated 300,000 people living in the United States today have CCM, of which its majority is undiagnosed. Wepresent a case of CCM acquired in an endemic area and detected in its early stage. A 42-year-old El Salvadoran woman presented with recurrent chest pain and syncopal episodes. Significant family history includes a sister inEl Salvador who also began suffering similar episodes. Physical exam and ancillary studies were only remarkable for sinus bradycardia. The patient was diagnosed with symptomatic sinus bradycardia and a pacemaker was placed. During her hospital course, Chagas serology was ordered given the epidemiological context from which she came. -
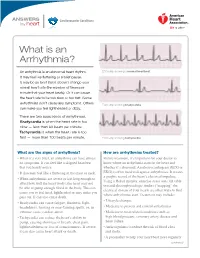
What Is an Arrhythmia?
ANSWERS Cardiovascular Conditions by heart What is an Arrhythmia? An arrhythmia is an abnormal heart rhythm. ECG strip showing a normal heartbeat It may feel like fluttering or a brief pause. It may be so brief that it doesn’t change your overall heart rate (the number of times per minute that your heart beats). Or it can cause the heart rate to be too slow or too fast. Some arrhythmias don’t cause any symptoms. Others ECG strip showing bradycardia can make you feel lightheaded or dizzy. There are two basic kinds of arrhythmias. Bradycardia is when the heart rate is too slow — less than 60 beats per minute. Tachycardia is when the heart rate is too fast — more than 100 beats per minute. ECG strip showing tachycardia What are the signs of arrhythmia? How are arrhythmias treated? • When it’s very brief, an arrhythmia can have almost Before treatment, it’s important for your doctor to no symptoms. It can feel like a skipped heartbeat know where an arrhythmia starts in the heart and that you barely notice. whether it’s abnormal. An electrocardiogram (ECG or • It also may feel like a fluttering in the chest or neck. EKG) is often used to diagnose arrhythmias. It creates a graphic record of the heart’s electrical impulses. • When arrhythmias are severe or last long enough to Using a Holter monitor, exercise stress tests, tilt table affect how well the heart works, the heart may not test and electrophysiologic studies (“mapping” the be able to pump enough blood to the body.