Immunopathology Jeffrey S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urticaria Is a Skin Condition Commonly Known As Hives. It Produces an Itchy Rash That Tends to Come and Go and Can Last for a Variable Period of Time
Hives Also known as Urticaria What is urticaria? Urticaria is a skin condition commonly known as hives. It produces an itchy rash that tends to come and go and can last for a variable period of time. The condition can be acute (lasting less than 6 weeks) or chronic (lasting longer than 6 weeks). Most cases of urticaria have no known cause. What causes urticaria? Urticaria occurs when certain substances such as histamine are released from specific cells in the skin. This process is usually triggered by various immunologic mechanisms, most commonly involving the presee of irulatig IgE atiodies, although other pathays ay also e ioled. The ause of this iuologi triggerig is uko i the ajority of ases, ut a soeties e associated with various types of infections, chronic immunologic diseases or allergy to foods or medications. The use of intravenous dyes during some radiological tests can sometimes trigger urticaria as well. Physical urticaria is a type of urticaria that may be caused by exposure of the skin to cold, heat, pressure or rubbing. What does urticaria look like? Urticaria typically looks like a raised rash that may be a normal skin colour or pinkish or red in colour. The rash may occur anywhere on the body and often starts off as small round spots that quickly enlarge and spread. The rash can be very itchy but it usually only lasts for a few hours before settling, and eventually resolving completely within 24 to 48 hours. Which other problems may occur with urticaria? A viral illness can occur before urticaria develops, especially in children. -

Four Diseases, PLAID, APLAID, FCAS3 and CVID and One Gene
Four diseases, PLAID, APLAID, FCAS3 and CVID and one gene (PHOSPHOLIPASE C, GAMMA-2; PLCG2 ) : striking clinical phenotypic overlap and difference Necil Kutukculer1, Ezgi Yilmaz1, Afig Berdeli1, Raziye Burcu G¨uven Bilgin1, Ayca Aykut1, Asude Durmaz1, Ozgur Cogulu1, G¨uzideAksu1, and Neslihan Karaca1 1Ege University Faculty of Medicine May 15, 2020 Abstract We suggest PLAID,APLAID and FCAS3 have to be considered as same diseases,because of our long-term clinical experiences and genetic results in six patients.Small proportion of CVID patients are also PLAID/APLAID/FCAS3 patients and all these have disease-causing-mutations in PLCG2-genes,so it may be better to define all of them as “PLCG2 deficiency”. Key Clinical Message: Germline mutations in PLCG2 gene cause PLAID,APLAID,FCAS3, and CVID.Clinical experiences in patients with PLCG2 mutations led us to consider that PLAID, APLAID and FCAS3 are same diseases.It may be better to define all of them as “PLCG2 deficiency”. INTRODUCTION The PLCG2 gene which is located on the 16th chromosome (16q23.3) encodes phospholipase Cg2 (PLCG2), a transmembrane signaling enzyme that catalyzes the production of second messenger molecules utilizing calcium as a cofactor and propagates downstream signals in several hematopoietic cells (1). Recently, het- erozygous germline mutations in human PLCG2 were linked to some clinical phenotypes with some overlap- ping features|PLCg2-associated antibody deficiency and immune dysregulation syndrome (PLAID) (OMIM 614878) and autoinflammation, antibody deficiency, and immune dysregulation syndrome (APLAID) (OMIM 614878) (2-4) and familial cold autoinflammatory syndrome (FCAS3) (OMIM 614468) (5). All of them are autosomal dominant inherited diseases. -

Open Access for Incredible Lupus Research
Lupus: Open Access Editorial Note Lupus: Open Access for Incredible Lupus Research Yves Renaudineau* Immunotherapy Graft Oncology, Innovative Medicines initiative precisesads, Réseau épigénétique et réseau canaux ioniques du Cancéropole Grand Ouest, European University of Brittany, Brest, France EDITOR’S PICKS The 04th Volume of this esteemed journal addresses the novel research performed by authors from parts of the world. Lupus is an Incurable auto immune disorder in which body Weinmann-Menke J, et al. in their case reports discusses that immune system attacks its own tissues or organs. It affects many Since our patient is suffering from the autoimmune disease parts of the body including Skin (Subcutaneous/cutaneous lupus erythematodes and is listed for kidney transplantation due Lupus), Kidneys (Lupus Nephrites), Brain (Cerebral/CNS to end stage renal disease, it is very likely that Lupus) etc., with several symptoms of Rashes (Malar, Discoid or immunosuppressant therapy will have to be initiated again in the photosensitive), Musculoskeletal Problems, Serositis, Anemia, future [1]. Seziures and several many more. We can find several Diagnostic approaches for Lupus. Elagib EM, et al. in his research article discloses that It is important to identify the possible differences in the effectiveness There is no cure for lupus, Yet many treatment options includes of rituximab in treating patients of CAPS associated with SLE NSAIDS, Immunosuoressives, Malario therapy, Usage of and patients of primary CAPS in order to determine the possible Steroids along with life style modifications and healthy diet can prognostic factors that could affect the therapeutic decisions and reduce the risk of lupus effected patients. The statistics provided results [2]. -

Immune Disorder
EDITORIAL Immune disorder Dr.Dingliang Lv* EDITORIAL vaccines and antiseptics, kids today aren’t exposed to as many germs as they were at intervals the past. The shortage of exposure might produce their Immune Disorder could also be a condition at intervals that your system system liable to react to harmless substances. mistakenly attacks your body. The system guards against germs like bacteria and viruses. Once it senses these foreign invaders, it sends out a military of The early symptoms of the many response diseases square measure terribly fighter cells to attack them. In degree illness, the system mistakes a section similar, such as: Fatigue, aching muscles, Swelling and redness, inferior of your body, like your joints or skin, as foreign. It releases proteins fever, bother concentrating, symptom and tingling within the hands and mentioned as auto antibodies that attack healthy cells. Some reaction feet, Hair loss, Skin rashes. diseases target only 1 organ. The system can tell the excellence between Individual diseases may also have their own distinctive symptoms. For foreign cells and your own cells. instance, kind one polygenic disorder causes extreme thirst, weight loss, and Immune System Attack the body because of the following reasons however fatigue. IBD causes belly pain, bloating, and looseness of the bowels. With some people square Measure plenty of probably to induce degree illness response diseases like skin condition or RA, symptoms might return and go. than others. In step with a 2014 study, girls get reaction diseases at a rate of An amount of symptoms is termed outburst. An amount once the regarding 2 to 1 compared to men-half-dozen.4 proportion of women vs. -

Hypersensitivity in General Immune System Is Protective in Nature by Protecting Host Body from Harmful and Infectious Foreign Microorganisms
Hypersensitivity In general immune system is protective in nature by protecting host body from harmful and infectious foreign microorganisms. But hypersensitivity is an immune disorder where immune system responds more than required and cause harmful effects in host. Hypersensitivity is defined as an exaggeration immune response that results in tissue damage in a sensitized individual on 2nd or subsequent contact with specific antigens. This condition is also called allergy. The first contact with an antigen leads to sensitization the immune system in the host for production of specific allergen produces the manifestation of hypersensitivity. This is known as shocking dose. Basing on the time required for the appearance of the hypersensitivity reactions. They are classified into two types: 1. Immediate hypersensitivity 2. Delayed hypersensitivity Peter Gell and Robert Coombs classified hypersensitivity reactions into 4 types based on the mechanism of pathogenesis: Type 1: IgE mediated hypersensitivity Type 2: Ab dependent cytotoxic hypersensitivity Type 3: Immune complex mediated hypersensitivity Type 4: Cell mediated delayed hypersensitivity The first three types of hypersensitivity reactions are mediated through the production of Antibodies and the fourth type of reaction is mediated through the production of T cells. Type 1: IgE mediated hypersensitivity It occurs in two forms anaphylaxis and atopy. i. Anaphylaxis It is an acute, fatal and systemic form of type I hypersensitivity. The allergens include antibiotics like Penicillin, antitoxin, insect venom etc. On first contact with allergen and lymphocytes are differentiated into plasma cells and produce specific Immunoglobulin IgE also called as ''Reagin antibodies'' which binds to the Fc receptors of mast cells or basophiles and sensitizes these cells that make the individual allergic to the antigen. -

Difference Between Hypersensitivity and Autoimmunity Key Difference – Hypersensitivity Vs Autoimmunity
Difference Between Hypersensitivity and Autoimmunity www.differencebetwee.com Key Difference – Hypersensitivity vs Autoimmunity Autoimmunity is an adaptive immune response mounted against self-antigens. In simple terms, when your body is acting against its own cells and tissues, this is called an autoimmune reaction. An exaggerated and inappropriate immune response to an antigenic stimulus is defined as a hypersensitivity reaction. Unlike autoimmune reactions that are triggered only by the endogenous antigens, hypersensitivity reactions are triggered by both endogenous and exogenous antigens. This is the key difference between hypersensitivity and autoimmunity. What is Hypersensitivity? An exaggerated and inappropriate immune response to an antigenic stimulus is defined as a hypersensitivity reaction. The first exposure to a particular antigen activates the immune system and, antibodies are produced as a result. This is called sensitization. Subsequent exposures to the same antigen give rise to hypersensitivity. Few important facts regarding the hypersensitivity reactions are given below They can be elicited by both exogenous and endogenous agents. They are a result of an imbalance between the effector mechanisms and the countermeasures that are there to control any inappropriate execution of an immune response. The presence of a genetic susceptibility increases the likelihood of hypersensitivity reactions. The manner in which hypersensitivity reactions harm our body is similar to the way that the pathogens are destroyed by immune reactions. Figure 01: Allergy According to the Coombs and Gell classification, there are four main types of hypersensitivity reactions. Type I- Immediate Type/ Anaphylactic Mechanism Vasodilation, edema, and contraction of smooth muscles are the pathological changes that take place during the immediate phase of the reaction. -

Ii Vasclitides
ا يمون وپات وژنز واسکوليتھااکلتا Vascliiitides: The Immunopa tho genes is دکتر محمدمھدی محمدی LMD, PhD, MPH ([email protected]) The 7th International 12th National Congress on Quality Improvement in Clinical Laboratories 28 Farvardin 1393 / 17April 2014 , Tehran, Iran A small spoonful of Terminology (in Farsi بيماريزايی) – .path·o·gen·e·sis, n • – the ppp(roduction and development of disease (events and reactions and other pathologic mechanisms) . Also, pa·thog·e·ny !?! (in Farsi بيماريزايی) _ .path·o·ge·nic·i·ty, n • – the disease-producing capacity of a pathogen. [PATHOGENIC + -ITY] • vir·u·lence, n. – the relative ability of a microorganism to cause disease; degree of pathogenicity. • Vasculitis, n. (vasculitides, plural) – a general term for vessel wall inflammation, systemic or localized, immune-mediated or directly invaded by microbes. • Infections may indirectly generate vasculitis, e.g. through IC formation or by X_reaction. Note that immunosuppressive therapy is only appropriate for --?– type! • WHAT R the IMMUNE MECHANISMS in VASCULITIS? افراط تعادل تفريط Immunodeficiency Allergy (& Infection) Defence Immunodeficiency Surveillance Autoimmunity (& Malignancy) Homostasis ?! Some Inter-related Concepts Immunological Vasculitis Hypersensitivity Auoimmunity Tolerance EDUCATION? Tolerance in different classic Txtbks of Immunology (Abbas, Benjamini, Janeway, Kuby, Roitt, Stites …) • Tolerance (by C&M Immunology): – Unresponsiveness of the adaptive immune system to antigens, as a result of inactivation or death of antigen-specific lymphocytes, induced by exposure to the antigens. – (()Active) Unresponsiveness of the (()HEALTHY) adappytive immune system to (selected) antigens, as a result of (i.e. MECHANISMS:) inactivation or death of antigen-specific lymphocytes, induced by exposure to those antigens. • Tolerance to self antigens is a normal feature of the adaptive immune system, but tolerance to foreign antigens may be induced undtiditiftider certain conditions of antigen exposure. -

Clinical Pathology, Immunopathology and Advanced Vaccine Technology in Bovine Theileriosis: a Review
pathogens Review Clinical Pathology, Immunopathology and Advanced Vaccine Technology in Bovine Theileriosis: A Review Onyinyechukwu Ada Agina 1,2,* , Mohd Rosly Shaari 3, Nur Mahiza Md Isa 1, Mokrish Ajat 4, Mohd Zamri-Saad 5 and Hazilawati Hamzah 1,* 1 Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] 2 Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, University of Nigeria Nsukka, Nsukka 410001, Nigeria 3 Animal Science Research Centre, Malaysian Agricultural Research and Development Institute, Headquarters, Serdang 43400, Malaysia; [email protected] 4 Department of Veterinary Pre-clinical sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] 5 Research Centre for Ruminant Diseases, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] * Correspondence: [email protected] (O.A.A.); [email protected] (H.H.); Tel.: +60-11-352-01215 (O.A.A.); +60-19-284-6897 (H.H.) Received: 2 May 2020; Accepted: 16 July 2020; Published: 25 August 2020 Abstract: Theileriosis is a blood piroplasmic disease that adversely affects the livestock industry, especially in tropical and sub-tropical countries. It is caused by haemoprotozoan of the Theileria genus, transmitted by hard ticks and which possesses a complex life cycle. The clinical course of the disease ranges from benign to lethal, but subclinical infections can occur depending on the infecting Theileria species. The main clinical and clinicopathological manifestations of acute disease include fever, lymphadenopathy, anorexia and severe loss of condition, conjunctivitis, and pale mucous membranes that are associated with Theileria-induced immune-mediated haemolytic anaemia and/or non-regenerative anaemia. -
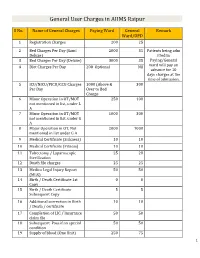
General User Charges in AIIMS Raipur
General User Charges in AIIMS Raipur S No. Name of General Charges Paying Ward General Remark Ward/OPD 1 Registration Charges 200 25 2 Bed Charges Per Day (Sami 2000 35 Patients being adm Deluxe) itted in 3 Bed Charges Per Day (Deluxe) 3000 35 Paying/General 4 Diet Charges Per Day 200 Optional Nil ward will pay an advance for 10 days charges at the time of admission. 5 ICU/NICU/PICU/CCU Charges 1000 (Above & 300 Per Day Over to Bed Charge 6 Minor Operation in OT/MOT 250 100 not mentioned in list, under L A 7 Minor Operation in OT/MOT 1000 300 not mentioned in list, under G A 8 Major Operation in OT, Not 2000 1000 mentioned in list under G A 9 Medical Certificate (Sickness) 10 10 10 Medical Certificate (Fitness) 10 10 11 Tubectomy / Laparoscopic 25 20 Sterilization 12 Death file charges 25 25 13 Medico Legal Injury Report 50 50 (MLR) 14 Birth / Death Certificate 1st 0 0 Copy 15 Birth / Death Certificate 5 5 Subsequent Copy 16 Additional correction in Birth 10 10 / Death / certificate 17 Completion of LIC / Insurance 50 50 claim file 18 Subsequent Pass if on special 50 50 condition 19 Supply of blood (One Unit) 250 75 1 20 Medical Board Certificate 500 500 On Special Case User Charges for Investigations in AIIMS Raipur S No. Name of Investigations Paying General Remark Ward Ward/OPD Anaesthsia 1 ABG 75 50 2 ABG ALONGWITH 150 100 ELECTROLYTES(NA+,K+)(Na,K) 3 ONLY ELECTROLYTES(Na+,K+,Cl,Ca+) 75 50 4 ONLY CALCIUM 50 25 5 GLUCOSE 25 20 6 LACTATE 25 20 7 UREA. -

Practice Parameter for the Diagnosis and Management of Primary Immunodeficiency
Practice parameter Practice parameter for the diagnosis and management of primary immunodeficiency Francisco A. Bonilla, MD, PhD, David A. Khan, MD, Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD, David I. Bernstein, MD, Joann Blessing-Moore, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Chief Editor: Francisco A. Bonilla, MD, PhD Co-Editor: David A. Khan, MD Members of the Joint Task Force on Practice Parameters: David I. Bernstein, MD, Joann Blessing-Moore, MD, David Khan, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Primary Immunodeficiency Workgroup: Chairman: Francisco A. Bonilla, MD, PhD Members: Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD GlaxoSmithKline, Merck, and Aerocrine; has received payment for lectures from Genentech/ These parameters were developed by the Joint Task Force on Practice Parameters, representing Novartis, GlaxoSmithKline, and Merck; and has received research support from Genentech/ the American Academy of Allergy, Asthma & Immunology; the American College of Novartis and Merck. -

Microbiology and Immunology
College of Medicine MI Microbiology and Immunology MI 494G IMMUNOBIOLOGY. (3) A survey of theories and mechanisms of immunity, including: nature of antigens and antibodies, antigen-antibody reactions, immunocompetent cells, immunogenetics, allergic reactions, tumor immunology and transplantation immunology. Prereq: BCH 401G (may be taken concurrently) and BIO 208 or BIO 308 or consent of instructor. (Same as BIO 494G.) MI 590 CELLULAR AND MOLECULAR PHYSIOLOGY. (4) This course will focus on the cellular and molecular physiology of inter-and intracellular communication. In particular, it will provide an overview of established and emerging intracellular signaling mechanisms which utilize i) cyclic nucleotides (cAMP; cGMP), ii) calcium (phosphatidylinositol metabolism: cyclic ADP-ribose), iii) transmembrane ion fluxes (voltage- and receptor-operated channels), iv) tyrosine kinases, and v) nuclear transcription factors. The material will be presented in a number of formats including didactic lecture and group discussions of selected readings. Prereq: PGY 412G, PGY 502 or consent of instructor. (Same as PGY 590.) MI 595 IMMUNOBIOLOGY LABORATORY. (2) Laboratory in immunology and serology. Preparation, standardization, and uses of biological products; serology. Laboratory; four hours. Prereq: BIO/MI 494G or concurrently; or consent of instructor. (Same as BIO 595.) MI 598 CLINICAL MICROBIOLOGY. (3) An introduction to the concepts of clinical microbiology through a survey of the microbial diseases of man using an organ system approach. Prereq: BIO 208 and 209, BIO 476G recommended, CHE 230 or 236, or consent of instructor. (Same as PAT 598.) MI 601 SPECIAL TOPICS IN MOLECULAR AND CELLULAR GENETICS. (1) Each semester five distinguished scientists visit the UK campus to deliver a series of three formal lectures each and participate in numerous informal contacts with graduate students. -

Hypersensitivity Reactions (Types I, II, III, IV)
Hypersensitivity Reactions (Types I, II, III, IV) April 15, 2009 Inflammatory response - local, eliminates antigen without extensively damaging the host’s tissue. Hypersensitivity - immune & inflammatory responses that are harmful to the host (von Pirquet, 1906) - Type I Produce effector molecules Capable of ingesting foreign Particles Association with parasite infection Modified from Abbas, Lichtman & Pillai, Table 19-1 Type I hypersensitivity response IgE VH V L Cε1 CL Binds to mast cell Normal serum level = 0.0003 mg/ml Binds Fc region of IgE Link Intracellular signal trans. Initiation of degranulation Larche et al. Nat. Rev. Immunol 6:761-771, 2006 Abbas, Lichtman & Pillai,19-8 Factors in the development of allergic diseases • Geographical distribution • Environmental factors - climate, air pollution, socioeconomic status • Genetic risk factors • “Hygiene hypothesis” – Older siblings, day care – Exposure to certain foods, farm animals – Exposure to antibiotics during infancy • Cytokine milieu Adapted from Bach, JF. N Engl J Med 347:911, 2002. Upham & Holt. Curr Opin Allergy Clin Immunol 5:167, 2005 Also: Papadopoulos and Kalobatsou. Curr Op Allergy Clin Immunol 7:91-95, 2007 IgE-mediated diseases in humans • Systemic (anaphylactic shock) •Asthma – Classification by immunopathological phenotype can be used to determine management strategies • Hay fever (allergic rhinitis) • Allergic conjunctivitis • Skin reactions • Food allergies Diseases in Humans (I) • Systemic anaphylaxis - potentially fatal - due to food ingestion (eggs, shellfish,