A Late Glacial-Early Holocene Paleoclimate Signal from the Ostracode
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Baseline Assessment of the Lake Ohrid Region - Albania
TOWARDS STRENGTHENED GOVERNANCE OF THE SHARED TRANSBOUNDARY NATURAL AND CULTURAL HERITAGE OF THE LAKE OHRID REGION Baseline Assessment of the Lake Ohrid region - Albania IUCN – ICOMOS joint draft report January 2016 Contents ........................................................................................................................................................................... i A. Executive Summary ................................................................................................................................... 1 B. The study area ........................................................................................................................................... 5 B.1 The physical environment ............................................................................................................. 5 B.2 The biotic environment ................................................................................................................. 7 B.3 Cultural Settings ............................................................................................................................ 0 C. Heritage values and resources/ attributes ................................................................................................ 6 C.1 Natural heritage values and resources ......................................................................................... 6 C.2 Cultural heritage values and resources....................................................................................... 12 D. -

Volume 2, Chapter 10-2: Arthropods: Crustacea
Glime, J. M. 2017. Arthropods: Crustacea – Ostracoda and Amphipoda. Chapt. 10-2. In: Glime, J. M. Bryophyte Ecology. Volume 2. 10-2-1 Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 10-2 ARTHROPODS: CRUSTACEA – OSTRACODA AND AMPHPODA TABLE OF CONTENTS CLASS OSTRACODA ..................................................................................................................................... 10-2-2 Adaptations ................................................................................................................................................ 10-2-3 Swimming to Crawling ....................................................................................................................... 10-2-3 Reproduction ....................................................................................................................................... 10-2-3 Habitats ...................................................................................................................................................... 10-2-3 Terrestrial ............................................................................................................................................ 10-2-3 Peat Bogs ............................................................................................................................................ 10-2-4 Aquatic ............................................................................................................................................... -

Crustacea, Ostracoda), from Christmas Island (Indian Ocean) with Some Considerations on the Morphological Evolution of Ancient Asexuals
Belg. J. Zool., 141 (2) : 55-74 July 2011 Description of a new genus and two new species of Darwinulidae (Crustacea, Ostracoda), from Christmas Island (Indian Ocean) with some considerations on the morphological evolution of ancient asexuals Giampaolo Rossetti1*, Ricardo L. Pinto 2 & Koen Martens 3 1 University of Panna, Department of Enviromnental Sciences, Viale G.P. Usberti 33 A, 1-43100 Panna, Italy 2 University of Brasilia, Institute of Geosciences, Laboratory of Micropaleontology, ICC, Campus Universitário Darcy Ribeiro Asa Norte, 70910-900 Brasilia, DF, Brazil 3 Royal Belgian Institute of Natural Sciences, Freshwater Biology, Vautierstraat 29, B-1000 Brussels, Belgium, and University of Ghent, Department of Biology, K.L. Ledeganckstraat 35, B-9000 Gent, Belgimn * Conesponding author: Giampaolo Rossetti. Mail: giampaolo.rosscttin unipr.it ABSTRACT. Darwinulidae is believed to be one of the few metazoan taxa in which fully asexual reproduction might have persisted for millions of years. Although rare males in a single darwinulid species have recently been found, they may be non-functional atavisms. The representatives of this family are characterized by a slow evolutionary rate, resulting in a conservative morphology in the different lineages over long time frames and across wide geographic ranges. Differences between species and genera, although often based on small details of valve morphology and chaetotaxy, are nevertheless well-recognizable. Five recent genera ( Darwinula, Alicenula, Vestcdemilct, Penthesilemila and Microdarwimda) and about 35 living species, including also those left in open nomenclature, are included in this family. Previous phylogenetic analyses using both morphological characters and molecular data confirmed that the five genera are good phyletic units. -

Development of the Eocene Elko Basin, Northeastern Nevada: Implications for Paleogeography and Regional Tectonism
DEVELOPMENT OF THE EOCENE ELKO BASIN, NORTHEASTERN NEVADA: IMPLICATIONS FOR PALEOGEOGRAPHY AND REGIONAL TECTONISM by SIMON RICHARD HAYNES B.Sc, Brock University, 1998 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES (Department of Earth and Ocean Sciences) We accept this thesis as conforming _tQ the required standard THE UNIVERSITY OF BRITISH COLUMBIA April 2003 © Simon Richard Haynes, 2003 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. < Department of \z~<xc ^Qp^rs SOA<S>C-> QS_2> The University of British Columbia Vancouver, Canada ABSTRACT Middle to late Eocene sedimentary and volcanic rocks in northeastern Nevada document the formation of broad lakes, two periods of crustal extension, and provide compelling evidence that the Carlin trend was a topographic high during a major phase of gold formation. The Eocene Elko Formation consists of alluvial-lacustrine rocks that were deposited into a broad, extensional basin between the present-day Ruby Mountains-East Humboldt Range metamorphic core complex and the Tuscarora Mountains. The rocks are divided into the lacustrine-dominated, longer-lived, eastern Elko Basin, and the alluvial braidplain facies of the shorter-lived western Elko Basin. -

Massachusetts Licensed Motor Vehicle Damage Appraisers - Individuals September 05, 2021
COMMONWEALTH OF MASSACHUSETTS DIVISION OF INSURANCE PRODUCER LICENSING 1000 Washington Street, Suite 810 Boston, MA 02118-6200 FAX (617) 753-6883 http://www.mass.gov/doi Massachusetts Licensed Motor Vehicle Damage Appraisers - Individuals September 05, 2021 License # Licensure Individual Address City State Zip Phone # 1 007408 01/01/1977 Abate, Andrew Suffolk AutoBody, Inc., 25 Merchants Dr #3 Walpole MA 02081 0-- 0 2 014260 11/24/2003 Abdelaziz, Ilaj 20 Vine Street Lexington MA 02420 0-- 0 3 013836 10/31/2001 Abkarian, Khatchik H. Accurate Collision, 36 Mystic Street Everett MA 02149 0-- 0 4 016443 04/11/2017 Abouelfadl, Mohamed N Progressive Insurance, 2200 Hartford Ave Johnston RI 02919 0-- 0 5 016337 08/17/2016 Accolla, Kevin 109 Sagamore Ave Chelsea MA 02150 0-- 0 6 010790 10/06/1987 Acloque, Evans P Liberty Mutual Ins Co, 50 Derby St Hingham MA 02018 0-- 0 7 017053 06/01/2021 Acres, Jessica A 0-- 0 8 009557 03/01/1982 Adam, Robert W 0-- 0 West 9 005074 03/01/1973 Adamczyk, Stanley J Western Mass Collision, 62 Baldwin Street Box 401 MA 01089 0-- 0 Springfield 10 013824 07/31/2001 Adams, Arleen 0-- 0 11 014080 11/26/2002 Adams, Derek R Junior's Auto Body, 11 Goodhue Street Salem MA 01970 0-- 0 12 016992 12/28/2020 Adams, Evan C Esurance, 31 Peach Farm Road East Hampton CT 06424 0-- 0 13 006575 03/01/1975 Adams, Gary P c/o Adams Auto, 516 Boston Turnpike Shrewsbury MA 01545 0-- 0 14 013105 05/27/1997 Adams, Jeffrey R Rodman Ford Coll Ctr, Route 1 Foxboro MA 00000 0-- 0 15 016531 11/21/2017 Adams, Philip Plymouth Rock Assurance, 901 Franklin Ave Garden City NY 11530 0-- 0 16 015746 04/25/2013 Adams, Robert Andrew Country Collision, 20 Myricks St Berkley MA 02779 0-- 0 17 013823 07/31/2001 Adams, Rymer E 0-- 0 18 013999 07/30/2002 Addesa, Carmen E Arbella Insurance, 1100 Crown Colony Drive Quincy MA 02169 0-- 0 19 014971 03/04/2008 Addis, Andrew R Progressive Insurance, 300 Unicorn Park Drive 4th Flr Woburn MA 01801 0-- 0 20 013761 05/10/2001 Adie, Scott L. -
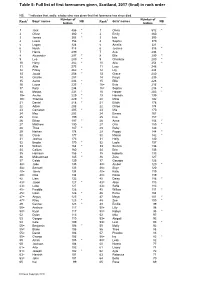
Table 5: Full List of First Forenames Given, Scotland, 2016 (Final) in Rank Order
Table 5: Full list of first forenames given, Scotland, 2017 (final) in rank order NB: * indicates that, sadly, a baby who was given that first forename has since died. Number of Number of Rank1 Boys' names NB Rank1 Girls' names NB babies babies 1 Jack 486 * 1 Olivia 512 * 2 Oliver 380 * 2 Emily 460 3 James 368 * 3 Isla 395 4 Lewis 356 4 Sophie 370 5 Logan 324 5 Amelia 321 6 Noah 318 6 Jessica 318 * 7 Harris 299 7 Ava 294 8 Alexander 297 * 8 Ella 290 * 9 Leo 289 * 9 Charlotte 280 * 10 Harry 282 * 10 Aria 254 * 11 Alfie 275 11 Lucy 248 12 Finlay 262 * 12 Lily 244 13 Jacob 258 * 13 Grace 240 14 Charlie 257 14 Freya 235 15 Aaron 236 * 15 Ellie 228 16 Lucas 235 * 16= Evie 216 17 Rory 234 16= Sophia 216 * 18 Mason 231 * 18 Harper 203 * 19= Archie 229 * 19 Hannah 199 19= Thomas 229 * 20 Millie 192 21 Daniel 218 * 21 Eilidh 178 22 Adam 208 22 Chloe 174 23 Cameron 205 * 23 Mia 170 24 Max 203 24 Emma 167 25 Finn 199 25 Eva 157 26 Ethan 197 * 26 Anna 154 * 27 Matthew 190 27 Orla 150 * 28 Theo 187 * 28 Ruby 146 29 Nathan 178 29 Poppy 144 * 30 Oscar 177 30 Maisie 142 * 31 Joshua 173 31 Holly 140 32 Brodie 170 * 32 Layla 137 33 William 164 * 33 Sienna 136 34 Callum 160 34 Erin 135 35 Harrison 156 * 35 Isabella 133 36 Muhammad 145 * 36 Zara 127 37 Caleb 139 37 Georgia 126 38= Jude 135 38= Amber 120 * 38= Samuel 135 38= Skye 120 40= Jamie 134 40= Katie 119 40= Ollie 134 40= Rosie 119 42 Liam 132 42 Daisy 116 * 43= Jaxon 127 * 43= Alice 113 43= Luke 127 43= Sofia 113 45= Freddie 126 45 Willow 111 * 45= Isaac 126 * 46 Esme 104 47= Angus 125 47 Maya 101 -

Ostracoda, Crustacea) in Turkey
LIMNOFISH-Journal of Limnology and Freshwater Fisheries Research 5(1): 47-59 (2019) Fossil and Recent Distribution and Ecology of Ancient Asexual Ostracod Darwinula stevensoni (Ostracoda, Crustacea) in Turkey Mehmet YAVUZATMACA * , Okan KÜLKÖYLÜOĞLU Department of Biology, Faculty of Arts and Science, Bolu Abant İzzet Baysal University, Turkey ABSTRACT ARTICLE INFO In order to determine distribution, habitat and ecological preferences of RESEARCH ARTICLE Darwinula stevensoni, data gathered from 102 samples collected in Turkey between 2000 and 2017 was evaluated. A total of 1786 individuals of D. Received : 28.08.2018 stevensoni were reported from eight different aquatic habitats in 14 provinces in Revised : 21.10.2018 six of seven geographical regions of Turkey. Although there are plenty of samples Accepted : 30.10.2018 from Central Anatolia Region, recent form of the species was not encountered. Unlike recent, fossil forms of species were encountered in all geographic regions Published : 25.04.2019 except Southeastern Anatolia. The oldest fossil record in Turkey was reported from the Miocene period (ca 23 mya). Species occurred in all climatic seasons in DOI:10.17216/LimnoFish.455722 Turkey. D. stevensoni showed high optimum and tolerance levels to different ecological variables. Results showed a positive and negative significant * CORRESPONDING AUTHOR correlations of the species with pH (P<0.05) and elevation (P<0.01), respectively. [email protected] It seems that the ecological preferences of the species are much wider than Phone : +90 537 769 46 28 previously known. Our results suggest that if D. stevensoni is used to estimate past and present environmental conditions, attention and care should be paid on its ecology and distribution. -

On Two New Species of Darwinula BRADY & ROBERTSON, 1885
BULLETIN DE L'INSTITUT ROYAL DES SCIENCES NATURELLES DE BELGIQUE, BIOLOGIE, 67: 57-66, 1997 '' BULLETIN VAN HET KONrNKLIJK BELGISCH INSTITUUT VOOR NATUURWETENSCHAPPEN, BIOLOGIE, 67: 57-66, 1997 On two new species of Darwinula BRADY & ROBERTSON, 1885 (Crustacea, Ostracoda) from South African dolomitic springs by Koen MARTENS & Giampaolo ROSSETTI Abstract 1968 (represented by only one extant spec1es, M. zimmeri) and the nominate genus Danvinula BRADY Two new Recent darwinulid ostrac ds (Darwinu/a molopoensis & ROB ERTSON, 1885. SOHN (1987) reported 23 living spec. nov. and D. inversa spec. nov.) are described from dolomitic species and 2 subspecies for Darwinula (D. dicastrii springs in the former North West Province (the former Transvaal), LOFFLER was missing from this list); amon·g these RSA. The two new taxa can be distinguished by both soft part species, only D. stevensoni can be considered truly and valve morphology. Darwinula molopoensis spec. nov. belongs ubiquitous. to the D. africana lineage (with D. incon5picua KuE as its Except for a few papers on D. stevensoni (McGREGOR & closest relative), D. inversa spec. nov. belongs into the D. serricaudata group. The synonymy of D. serricaudata espinosa WETZEL 1968, MCGREGOR 1969; RANTA, 1979), little is PINTO & KOTZIAN, 1961 with D. serricaudata KLIE, 1935 is known on the biology and ecology of the Darwinuloidea. discussed. Also taxonomic relationships within this group remain Key words: Ostracods, Darwinu/a mo/opoensis spec. nov., " "unclear, in s'pite of valuable contributions by DAN IELOPOL Darwinula inversa spec. nov., morphology, taxonomy, ancient (1968, 1970, 1980). Indeed, the morphological uniformity asexuals, parthenogenesis, biodiversity. of the Darwinuloidea makes it difficult to single out unequivocal characters suitable for discriminating species and genera. -

Late Pleistocene to Recent Ostracod Assemblages from the Western Black Sea Ian Boomer, Francois Guichard, Gilles Lericolais
Late Pleistocene to recent ostracod assemblages from the western Black Sea Ian Boomer, Francois Guichard, Gilles Lericolais To cite this version: Ian Boomer, Francois Guichard, Gilles Lericolais. Late Pleistocene to recent ostracod assemblages from the western Black Sea. Journal of Micropalaeontology, Geological Society, 2010, 29 (2), pp.119- 133. 10.1144/0262-821X10-003. hal-03199895 HAL Id: hal-03199895 https://hal.archives-ouvertes.fr/hal-03199895 Submitted on 1 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Journal of Micropalaeontology, 29: 119–133. 0262-821X/10 $15.00 2010 The Micropalaeontological Society Late Pleistocene to Recent ostracod assemblages from the western Black Sea IAN BOOMER1,*, FRANCOIS GUICHARD2 & GILLES LERICOLAIS3 1School of Geography, Earth & Environmental Sciences, University of Birmingham Birmingham B15 2TT, UK 2Laboratoire des Sciences du Climat et de l’Environnement (LSCE, CEA-CNRS-UVSQ) Avenue de la Terrasse, 91198 Gif sur Yvette, France 3IFREMER, Centre de Brest, Géosciences Marines, Laboratoire Environnements Sédimentaires BP70, F-29280 Plouzané cedex, France *Corresponding author (e-mail: [email protected]) ABSTRACT – During the last glacial phase the Black Sea basin was isolated from the world’s oceans due to the lowering of global sea-levels. -

Baby Girl Names | Registered in 2019
Vital Statistics Baby2019 BabyGirl GirlsNames Names | Registered in 2019 From:Jan 01, 2019 To: Dec 31, 2019 First Name Frequency First Name Frequency First Name Frequency Aadhini 1 Aarnavi 1 Aadhirai 1 Aaro 1 Aadhya 2 Aarohi 2 Aadila 1 Aarora 1 Aadison 1 Aarushi 1 Aadroop 1 Aarya 3 Aadya 3 Aarza 2 Aafiya 1 Aashvee 1 Aaghnya 1 Aasiya 1 Aahana 2 Aasiyah 2 Aaila 1 Aasmi 1 Aaira 3 Aasmine 1 Aaleena 1 Aatmja 1 Aalia 1 Aatri 1 Aaliah 1 Aayah 2 Aalis 1 Aayara 1 Aaliya 1 Aayat 1 Aaliyah 17 Aayla 1 Aaliyah-Lynn 1 Aayna 1 Aalya 1 Aayra 2 Aamina 1 Aazeen 1 Aamna 1 Abagail 1 Aanaya 1 Abbey 4 Aaniya 1 Abbi 1 Aaniyah 1 Abbie 1 Aanya 3 Abbiejean 1 Aara 1 Abbigail 3 Aaradhya 1 Abbiteal 1 Aaradya 1 Abby 10 Aaraya 1 Abbygail 1 Aaria 2 Abdirashid 1 Aariya 1 Abeeha 1 Aariyah 1 Aberlene 1 Aarna 3 Abhideep 1 Abi 1 Abiah 1 10 Jun 2020 1 Abiegail 02:22:21 PM1 Abigael 1 Abigail 141 Abigale 1 1 10 Jun 2020 2 02:22:21 PM Baby Girl Names | Registered in 2019 First Name Frequency First Name Frequency Abigayle 1 Addalyn 4 Abihail 1 Addalynn 1 Abilene 2 Addasyn 1 Abina 1 Addelyn 1 Abisha 2 Addi 1 Ablakat 1 Addie 2 Aboni 1 Addilyn 3 Abrahana 1 Addilynn 2 Abreen 1 Addison 61 Abrielle 2 Addisyn 2 Absalat 1 Addley 1 Abuk 2 Addy 1 Abyan 1 Addyson 2 Abygale 1 Adedoyin 1 Acadia 1 Adeeva 1 Acelynn 1 Adeifeoluwa 1 Achai 1 Adela 1 Achan 1 Adélaïde 1 Achol 1 Adelaide 20 Ackley 1 Adelaine 1 Ada 23 Adelayne 1 Adabpreet 1 Adele 4 Adaeze 1 Adèle 2 Adah 1 Adeleigha 1 Adair 1 Adeleine 1 Adalee 1 Adelheid 1 Adalena 1 Adelia 3 Adaley 1 Adelina 2 Adalina 2 Adeline 40 Adalind 1 Adella -

Table 4: Full List of First Forenames Given, Scotland, 2016 (Final) In
Table 4: Full list of first forenames given, Scotland, 2017 (final) in alphabetical order NB: * indicates that, sadly, a baby who was given that first forename has since died. Number of Number of Boys' names NB Girls' names NB babies babies A-Jay 2 Aabha 1 Aadam 4 Aadhya 1 Aaden 2 Aadhyaa 1 Aadhan 1 Aadia 1 Aadhith 1 Aadvika 1 Aadhrith 1 Aahliyah 1 Aadi 2 Aaida 1 Aadil 1 Aaila 1 Aadirath 1 Aailah 1 Aadrith 1 Aairah 5 Aahil 3 Aaishah 1 Aakin 1 Aaiylah 1 Aamir 2 Aaiza 1 Aaraf 1 Aakifa 1 Aaran 7 Aalayah 1 * Aarav 1 Aaleen 1 Aarif 1 Aaleyah 1 Aariv 1 Aalia 1 Aariz 2 Aaliya 1 Aaron 236 * Aaliyah 25 Aarron 6 Aaliyah-Louise 1 Aarush 4 Aaliyah-Mae 1 Aarya 1 Aaliyah-Raven 1 Aaryan 6 Aaliyah-Rose 1 Aaryn 3 Aamaya 1 Aaryn-Paul 1 Aara 1 Aashir 1 Aaria 3 Aayan 6 Aariah 2 Aayden 1 Aariyah 2 Aayden-James 1 Aarla 1 Aayden-Jon 1 Aarna 1 Aayush 1 Aarya 1 Abbas 2 Aathera 1 Abbdelrahmane 1 Aava 1 Abdalla 1 Aayat 3 Abdallah 2 Aayla 2 Abdelkrim 1 Abbey 4 Abdelrahman 1 Abbey-Leigh 1 Abderrahman 1 Abbi 4 Abderraouf 1 Abbie 52 Abdilahi 1 Abbie-Lee 1 Abdimalik 1 Abbie-Leigh 1 Abdoulie 1 Abbiegale 1 Abdul 8 Abby 19 Abdul-Haadi 1 Abby-Lily 1 Abdul-Hadi 1 Abeera 1 Abdul-Muizz 1 Aberdeen 1 Abdulfattah 1 Abi 7 Abduljabaar 1 Abigail 93 Abdullah 12 Abiha 3 Abdulmohsen 1 Abii 1 Abdulrahman 2 Abony 1 Abdulshakur 1 Abrish 1 Abdur-Rahman 2 Accalia 1 Abdurahman 1 Ada 33 Abdurrahman 1 Ada-Ellen 1 © Crown Copyright 2018 Table 4 (continued) NB: * indicates that, sadly, a baby who was given that first forename has since died Number of Number of Boys' names NB Girls' names NB -

Board of Commissioners Meeting Tuesday, June 28, 2016
City of Henderson, Kentucky Board of Commissioners Meeting Tuesday, June 28, 2016 Municipal Center Third Floor Assembly Room 222 First Street 5:30 P.M. AGENDA 1. Invocation: Reverend Mary Wrye, Methodist Hospital Chaplain 2. Roll Call: 3. Recognition of Visitors: 4. Appearance of Citizens: 5. Proclamations: "World Changers Week" 6. Presentations: 7. Public Hearings: 8. Consent Agenda: Minutes: June 14, 2016 Regular Meeting Resolutions: a. Resolution Authorizing Submittal of Grant Application to the Kentucky Office of Homeland Security (KOHS) for Funds in the Amount of $280,000.00 to Purchase New 911 Phone System, and Acceptance of Grant if Awarded b. Resolution Adopting and Approving Execution of Municipal Road Aid Cooperative Program Agreement for FY 2017 c. Resolution Approving Agreement with the Henderson City County Airport Board Allocating $135,336.00 for Airport Services d. Resolution Approving Agreement with the Downtown Henderson Partnership Allocating $46,000.00 for Services in Support of Downtown Henderson Please mute or turn off all cell phones for the duration of this meeting. e. Resolution Approving Agreement with Kentucky Network for Development, Leadership and Engagement, Inc. (Kyndle) Allocating $60,000.00 for Economic Development Services f. Resolution Approving Agreement with the Humane Society of Henderson County, Inc. Allocating $9,166.67 on a Monthly Basis for Animal Control and Shelter Services g. Resolution Approving Memorandum of Understanding Between the City of Henderson and Henderson County Tourist Commission Regarding Personnel for the Henderson Welcome Center h. Resolution Approving Community Development Block Grant Subrecipient Agreement with the Father Bradley Shelter for Women and Children, Inc. i.