Bulk Gold Catalyzes Hydride Transfer in the Cannizzaro and Related Reactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Yet Another Web Server
Yaws - Yet Another Web Server Claes Wikstrom [email protected] September 9, 2018 Contents 1 Introduction 4 1.1 Prerequisites . 5 1.2 A tiny example . 5 2 Compile, Install, Config and Run 7 2.0.1 Compile and Install . 7 2.0.2 Configure . 8 3 Static content 11 4 Dynamic content 12 4.1 Introduction . 12 4.2 EHTML . 12 4.3 POSTs . 17 4.3.1 Queries . 17 4.3.2 Forms . 17 4.4 POSTing files . 18 5 Mode of operation 22 5.1 On-the-fly compilation . 22 5.2 Evaluating the Yaws Code . 23 6 SSL 24 6.1 Server Name Indication . 25 7 Applications 26 7.1 Login scenarios . 26 7.1.1 The session server . 26 1 CONTENTS 2 7.1.2 Arg rewrite . 28 7.1.3 Authenticating . 29 7.1.4 Database driven applications . 31 7.2 Appmods . 31 7.3 The opaque data . 32 7.4 Customizations . 32 7.4.1 404 File not found . 33 7.4.2 Crash messages . 33 7.5 Stream content . 34 7.6 All out/1 Return Values . 35 8 Debugging and Development 39 8.1 Logs . 39 9 External scripts via CGI 40 10 FastCGI 41 10.1 The FastCGI Responder Role . 41 10.2 The FastCGI Authorizer Role . 42 10.3 The FastCGI Filter Role . 42 10.4 FastCGI Configuration . 42 11 Security 43 11.1 WWW-Authenticate . 43 12 Embedded mode 45 12.1 Creating Global and Server Configurations . 45 12.2 Starting Yaws in Embedded Mode . 46 13 The config file - yaws.conf 47 13.1 Global Part . -

SDK De AWS Para Ruby Developer Guide
SDK de AWS para Ruby Developer Guide SDK de AWS para Ruby: Developer Guide Copyright © Amazon Web Services, Inc. and/or its affiliates. All rights reserved. SDK de AWS para Ruby Developer Guide Las marcas comerciales y la imagen comercial de Amazon no se pueden utilizar en relación con ningún producto o servicio que no sea de Amazon de ninguna manera que pueda causar confusión entre los clientes y que menosprecie o desacredite a Amazon. Todas las demás marcas comerciales que no son propiedad de Amazon son propiedad de sus respectivos propietarios, que pueden o no estar afiliados, conectados o patrocinados por Amazon. SDK de AWS para Ruby Developer Guide Table of Contents AWSGuía para desarrolladores de SDK for Ruby ................................................................................... 1 Mediante laAWSSDK for Ruby conAWS Cloud9 .............................................................................. 1 Acerca de esta guía ................................................................................................................... 1 Documentación y recursos adicionales .......................................................................................... 2 Implementación enAWSCloud ............................................................................................... 2 Mantenimiento y soporte para las versiones principales del SDK ........................................................ 2 Introducción ...................................................................................................................................... -

85324630.Pdf
About NetTantra NetTantra is a creative technology and design company based out of India, US and UK. We provide web based solutions and mobile solutions to various industries like manufacturing, consulting, education. We have expertise in various sectors of the web including an array of server-side languages, OpenSource CMS/Blog frameworks, Linux/UNIX system administration, production server backup and recovery solutions, cloud infrastructure set-up and much more. Our expertise in providing WordPress based solutions has been acclaimed by many of our clients and the OpenSource community. We also provide cloud based solutions like migrating existing applications and building cloud applications for public or private cloud setups. We are known among our clients for on-time delivery and extraordinary quality of service. In mobile based solutions, we have expertise in developing native applications for iOS and Android platforms. We also develop cross-platform mobile applications using Sencha Touch and jQuery Mobile frameworks. 2 of 14 pages Why Hire Us ✔ Technology ◦ We have expertise in the most cutting edge tools and technologies used in the industry with special focus on OpenSource Technologies ◦ We pay special attention to web and network security for all projects ◦ Our team follows highly optimized project delivery life cycles and processes ✔ Cost ◦ We offer the best price to quality ratio ✔ Infrastructure ◦ Advanced workstations ◦ Cutting edge computing and network systems ◦ Power packed online servers ◦ Smart communications systems ◦ Conference halls, CBT and video learning facilities ◦ High-speed uninterrupted Internet connection ✔ Quality of Service ◦ Guaranteed client satisfaction ◦ Real-time customer support with the least turn-around in the industry ◦ Pre-sales technical and business related support to partners and agencies ✔ Ethics and Principles ◦ We ensure confidentiality in all our dealings. -

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

Direct Visualization of a Mechanochemically Induced Molecular Rearrangement Karen J
Angewandte Communications Chemie How to cite: Angew. Chem. Int. Ed. 2020, 59, 13458–13462 Mechanochemical Synthesis International Edition: doi.org/10.1002/anie.201914921 German Edition: doi.org/10.1002/ange.201914921 Direct Visualization of a Mechanochemically Induced Molecular Rearrangement Karen J. Ardila-Fierro, Stipe Lukin, Martin Etter, Krunoslav Uzˇarevic´, Ivan Halasz, Carsten Bolm, and JosØ G. Hernµndez* Abstract: Recent progress in the field of mechanochemistry (thio)acylations[5]). In this context, we became curious as to has expanded the discovery of mechanically induced chemical whether a combination of in situ real-time monitoring by transformations to several areas of science. However, a general synchrotron powder X-ray diffraction (PXRD), Raman fundamental understanding of how mechanochemical reac- spectroscopy, and temperature sensing could be applied for tions by ball milling occur has remained unreached. For this, the investigation of a fundamentally different type of organic we have now implemented in situ monitoring of a mechano- reaction; namely, a molecular rearrangement. As the first chemically induced molecular rearrangement by synchrotron example, we considered the emblematic benzil–benzilic acid X-ray powder diffraction, Raman spectroscopy, and real-time rearrangement in the presence of hydroxide ions (Sche- temperature sensing. The results of this study demonstrate that me 1a). molecular rearrangements can be accomplished in the solid state by ball milling and how in situ monitoring techniques enable the visualization of changes occurring at the exact instant of a molecular migration. The mechanochemical benzil–benzilic acid rearrangement is the focal point of the study. In mechanochemical reactions by ball milling, collisions of accelerated balls inside a milling container maintain a con- stant mixing of the reactants while simultaneously trans- ducing the energy required to induce specific physicochemical changes into the milled system. -

Next Generation Web Scanning Presentation
Next generation web scanning New Zealand: A case study First presented at KIWICON III 2009 By Andrew Horton aka urbanadventurer NZ Web Recon Goal: To scan all of New Zealand's web-space to see what's there. Requirements: – Targets – Scanning – Analysis Sounds easy, right? urbanadventurer (Andrew Horton) www.morningstarsecurity.com Targets urbanadventurer (Andrew Horton) www.morningstarsecurity.com Targets What does 'NZ web-space' mean? It could mean: •Geographically within NZ regardless of the TLD •The .nz TLD hosted anywhere •All of the above For this scan it means, IPs geographically within NZ urbanadventurer (Andrew Horton) www.morningstarsecurity.com Finding Targets We need creative methods to find targets urbanadventurer (Andrew Horton) www.morningstarsecurity.com DNS Zone Transfer urbanadventurer (Andrew Horton) www.morningstarsecurity.com Find IP addresses on IRC and by resolving lots of NZ websites 58.*.*.* 60.*.*.* 65.*.*.* 91.*.*.* 110.*.*.* 111.*.*.* 113.*.*.* 114.*.*.* 115.*.*.* 116.*.*.* 117.*.*.* 118.*.*.* 119.*.*.* 120.*.*.* 121.*.*.* 122.*.*.* 123.*.*.* 124.*.*.* 125.*.*.* 130.*.*.* 131.*.*.* 132.*.*.* 138.*.*.* 139.*.*.* 143.*.*.* 144.*.*.* 146.*.*.* 150.*.*.* 153.*.*.* 156.*.*.* 161.*.*.* 162.*.*.* 163.*.*.* 165.*.*.* 166.*.*.* 167.*.*.* 192.*.*.* 198.*.*.* 202.*.*.* 203.*.*.* 210.*.*.* 218.*.*.* 219.*.*.* 222.*.*.* 729,580,500 IPs. More than we want to try. urbanadventurer (Andrew Horton) www.morningstarsecurity.com IP address blocks in the IANA IPv4 Address Space Registry Prefix Designation Date Whois Status [1] ----- -

A Facile Solvent-Free Cannizzaro Reaction. an Instructional Model for Introductory Organic Chemistry Laboratory
In the Laboratory edited by Green Chemistry Mary M. Kirchhoff ACS Education Division Washington, DC 20036 A Facile Solvent-Free Cannizzaro Reaction An Instructional Model for Introductory Organic Chemistry Laboratory Sonthi Phonchaiya and Bhinyo Panijpan Institute for Innovation and Development of Learning Process, Mahidol University, Bangkok 10400, Thailand Shuleewan Rajviroongit* Department of Chemistry, Faculty of Science, Mahidol University, Bangkok 10400, Thailand; *[email protected] Tony Wright School of Education, University of Queensland, 4072, QLD, Australia Joanne T. Blanchfield School of Molecular and Microbial Sciences, University of Queensland, 4072, QLD, Australia To make students in chemistry laboratory more apprecia- 2-chlorobenzyl alcohol by filtration: the solid 2-chlorobenzyl tive of the environmental aspects of chemistry, it is important alcohol retained by the filter was washed twice with water. After that the laboratory exercise address possible environmental im- acidification of potassium 2-chlorobenzoate and filtration, the pacts, for example, energy used, chemical wastes produced, and precipitated 2-chlorobenzoic acid was also rinsed with water. In the use of organic solvents. A reaction that involves only a small both cases small amounts of products were sacrificed, leading to amount(s) of reactants in an aqueous solvent with no organic slightly reduced yields. The reaction is shown in Scheme I. solvent used in the product purification step would result in a Students achieved yields of 35–85% for each pure product. greener chemistry laboratory. A pedagogical method such as They characterized their partially dried products by comparing guided inquiry will make the laboratory more learner-centered the Rf values on TLC with the authentic samples. -

Practical Organic Pharmaceutical Chemistry 4Th Stage (2Nd Course)
Al-ISRAA University College Pharmacy Department ------------------------------------------------------------------------------------------------------------------------ Practical Organic Pharmaceutical Chemistry 4th Stage (2nd course) Lab. 1,2 Prepared by: Assist.Lecturer Ali Amjed 2017-2018 1 Al-ISRAA University College Pharmacy Department ------------------------------------------------------------------------------------------------------------------------ Cannizzaro Reaction Synthesis of Benzoic Acid and Benzyl Alcohol Both Alcohols and Organic Acids are Well Known for their Biological Actions which include: Antibacterial, Preservatives for Food and Pharmaceutics, Local Antiseptic, Local Anesthetic and Antipruritic. Benzyl Alcohol: Benzyl Alcohol can be Prepared by Hydrolysis of Benzyl Chloride with Sodium Hydroxide as shown below: Benzyl chloride Benzyl alcohol Properties of Benzyl Alcohol: 1) Colorless Liquid. 2) Moderate Solubility in Water. 3) Completely Miscible with Organic Solvents like Alcohol and Diethyl ether. 4) Boiling Point: 204-205 °C. 5) Have Low Vapor Pressure and Low Toxicity. 2 Al-ISRAA University College Pharmacy Department ------------------------------------------------------------------------------------------------------------------------ In Pharmaceutical , Benzyl Alcohol used at Low Concentration as: 1) Antiseptic (Bacterio Static). 2) Local Anesthetic. 3) Preservative for Food Industry. 4) In 10% Ointments is used as Antipruritic. In High Concentration Could Cause Hypotension and Respiratory Failure. Benzoic -

Pipenightdreams Osgcal-Doc Mumudvb Mpg123-Alsa Tbb
pipenightdreams osgcal-doc mumudvb mpg123-alsa tbb-examples libgammu4-dbg gcc-4.1-doc snort-rules-default davical cutmp3 libevolution5.0-cil aspell-am python-gobject-doc openoffice.org-l10n-mn libc6-xen xserver-xorg trophy-data t38modem pioneers-console libnb-platform10-java libgtkglext1-ruby libboost-wave1.39-dev drgenius bfbtester libchromexvmcpro1 isdnutils-xtools ubuntuone-client openoffice.org2-math openoffice.org-l10n-lt lsb-cxx-ia32 kdeartwork-emoticons-kde4 wmpuzzle trafshow python-plplot lx-gdb link-monitor-applet libscm-dev liblog-agent-logger-perl libccrtp-doc libclass-throwable-perl kde-i18n-csb jack-jconv hamradio-menus coinor-libvol-doc msx-emulator bitbake nabi language-pack-gnome-zh libpaperg popularity-contest xracer-tools xfont-nexus opendrim-lmp-baseserver libvorbisfile-ruby liblinebreak-doc libgfcui-2.0-0c2a-dbg libblacs-mpi-dev dict-freedict-spa-eng blender-ogrexml aspell-da x11-apps openoffice.org-l10n-lv openoffice.org-l10n-nl pnmtopng libodbcinstq1 libhsqldb-java-doc libmono-addins-gui0.2-cil sg3-utils linux-backports-modules-alsa-2.6.31-19-generic yorick-yeti-gsl python-pymssql plasma-widget-cpuload mcpp gpsim-lcd cl-csv libhtml-clean-perl asterisk-dbg apt-dater-dbg libgnome-mag1-dev language-pack-gnome-yo python-crypto svn-autoreleasedeb sugar-terminal-activity mii-diag maria-doc libplexus-component-api-java-doc libhugs-hgl-bundled libchipcard-libgwenhywfar47-plugins libghc6-random-dev freefem3d ezmlm cakephp-scripts aspell-ar ara-byte not+sparc openoffice.org-l10n-nn linux-backports-modules-karmic-generic-pae -
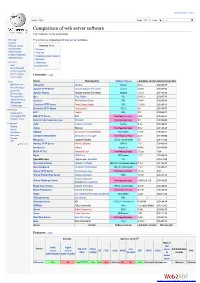
Comparison of Web Server Software from Wikipedia, the Free Encyclopedia
Create account Log in Article Talk Read Edit ViewM ohrisetory Search Comparison of web server software From Wikipedia, the free encyclopedia Main page This article is a comparison of web server software. Contents Featured content Contents [hide] Current events 1 Overview Random article 2 Features Donate to Wikipedia 3 Operating system support Wikimedia Shop 4 See also Interaction 5 References Help 6 External links About Wikipedia Community portal Recent changes Overview [edit] Contact page Tools Server Developed by Software license Last stable version Latest release date What links here AOLserver NaviSoft Mozilla 4.5.2 2012-09-19 Related changes Apache HTTP Server Apache Software Foundation Apache 2.4.10 2014-07-21 Upload file Special pages Apache Tomcat Apache Software Foundation Apache 7.0.53 2014-03-30 Permanent link Boa Paul Phillips GPL 0.94.13 2002-07-30 Page information Caudium The Caudium Group GPL 1.4.18 2012-02-24 Wikidata item Cite this page Cherokee HTTP Server Álvaro López Ortega GPL 1.2.103 2013-04-21 Hiawatha HTTP Server Hugo Leisink GPLv2 9.6 2014-06-01 Print/export Create a book HFS Rejetto GPL 2.2f 2009-02-17 Download as PDF IBM HTTP Server IBM Non-free proprietary 8.5.5 2013-06-14 Printable version Internet Information Services Microsoft Non-free proprietary 8.5 2013-09-09 Languages Jetty Eclipse Foundation Apache 9.1.4 2014-04-01 Čeština Jexus Bing Liu Non-free proprietary 5.5.2 2014-04-27 Galego Nederlands lighttpd Jan Kneschke (Incremental) BSD variant 1.4.35 2014-03-12 Português LiteSpeed Web Server LiteSpeed Technologies Non-free proprietary 4.2.3 2013-05-22 Русский Mongoose Cesanta Software GPLv2 / commercial 5.5 2014-10-28 中文 Edit links Monkey HTTP Server Monkey Software LGPLv2 1.5.1 2014-06-10 NaviServer Various Mozilla 1.1 4.99.6 2014-06-29 NCSA HTTPd Robert McCool Non-free proprietary 1.5.2a 1996 Nginx NGINX, Inc. -

Paper II, Organic Chemistry, Unit IV the Cannizzaro Reaction
B.Sc. Part-1(Hons.), Paper II, Organic Chemistry, unit IV (Study material prepared by Dr. Ruchi Jain, S.B.S.S.College, Begusarai) The Cannizzaro reaction The Cannizzaro reaction is also known as Cannizzaro disproportionation and it is a redox reaction between aromatic aldehydes, formaldehyde or other aliphatic aldehydes without α-hydrogen. Base is used to afford the corresponding alcohols and carboxylic acids.(pathway A) Final deprotonation of the carboxylic acid drives the reaction forward. Pathway B: As you know, aldehydes are generally at least partly hydrated in water. Hydration is catalysed by base, and we can represent the hydration step in base like this. The hydration product is an anion but, if the base is sufficiently strong (or concentrated) and as long as the aldehyde cannot be enolized, at least some will be present as a dianion. The dianion is very unstable, and one way in which it can become much more stable is by behaving like a tetrahedral intermediate. Which is the best leaving group? Out of a choice of O2–, R–, and H–, it’s H– that (if reluctantly) has to go. Hydride is, of course, too unstable to be released into solution but, if there is a suitable electrophile at hand (another molecule of aldehyde, for example), it is transferred to the electrophilic centre in a mechanism that bears some resemblance to a borohydride reduction. The dianion becomes a much more stable carboxylate monoanion, and a second molecule of aldehyde has been reduced to an alcohol. This is the Cannizzaro reaction: in this case it takes the form of a disproportionation of two molecules of aldehyde to one of carboxylate and one of alcohol. -

Aldol Condensation
SEM-III, CORE COURSE-7 ORGANIC CHEMISTRY-3 TOPIC: CARBONYL AND RELATED COMPOUNDS SUB-TOPIC: CONDENSATIONS (PPT-7) Dr. Kalyan Kumar Mandal Associate Professor St. Paul’s C. M. College Kolkata CONDENSATION • ALDOL CONDENSATION • CLAISEN-SCHMIDT REACTION • TOLLENS’ CONDENSATION ALDOL CONDENSATION • Carbonyl compounds, aldehydes and ketones, containing an α-hydrogen atom, in presence of dilute alkali, like NaOH, KOH, Ba(OH)2, etc., or dilute acids, like HCl, H2SO4 undergo a type of reaction in which two molecules of the aldehydes or ketones condense to form an unstable syrupy liquid, aldol. • This reaction is a dimerisation of an aldehyde (or ketone) to a β-hydroxyaldehyde (or β-hydroxyketone) by alpha C-H addition of one reactant molecule to the carbonyl group of the second reactant molecule. FACTS • The product is an aldehyde with a hydroxy (ol) group whose trivial name is aldol. The name aldol is given to the whole class of reactions between enolates (or enols) and carbonyl compounds even if the product is not a hydroxyaldehyde. • The Aldol Condensation leads to the formation of carbon-carbon sigma bond between two carbonyl compounds (same or different), one acting as a nucleophilic species and another as an electrophile. • The rate expression for the aldol reaction at low concentrations of - hydroxide ion is, rate = k [CH3CHO][HO ], showing that the formation of the enolate ion is rate-determining. • The base catalysed aldol condensation of acetaldehyde takes place in two steps, the first step being the formation of the carbanion (I), and the second, the combination of this anion with a second molecule of acetaldehyde to form the anion (II) of the aldol.