Predicting Microbiome Compositions Through Deep Learning
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microbial Community Structure Dynamics in Ohio River Sediments During Reductive Dechlorination of Pcbs
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2008 MICROBIAL COMMUNITY STRUCTURE DYNAMICS IN OHIO RIVER SEDIMENTS DURING REDUCTIVE DECHLORINATION OF PCBS Andres Enrique Nunez University of Kentucky Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Nunez, Andres Enrique, "MICROBIAL COMMUNITY STRUCTURE DYNAMICS IN OHIO RIVER SEDIMENTS DURING REDUCTIVE DECHLORINATION OF PCBS" (2008). University of Kentucky Doctoral Dissertations. 679. https://uknowledge.uky.edu/gradschool_diss/679 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Andres Enrique Nunez The Graduate School University of Kentucky 2008 MICROBIAL COMMUNITY STRUCTURE DYNAMICS IN OHIO RIVER SEDIMENTS DURING REDUCTIVE DECHLORINATION OF PCBS ABSTRACT OF DISSERTATION A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Agriculture at the University of Kentucky By Andres Enrique Nunez Director: Dr. Elisa M. D’Angelo Lexington, KY 2008 Copyright © Andres Enrique Nunez 2008 ABSTRACT OF DISSERTATION MICROBIAL COMMUNITY STRUCTURE DYNAMICS IN OHIO RIVER SEDIMENTS DURING REDUCTIVE DECHLORINATION OF PCBS The entire stretch of the Ohio River is under fish consumption advisories due to contamination with polychlorinated biphenyls (PCBs). In this study, natural attenuation and biostimulation of PCBs and microbial communities responsible for PCB transformations were investigated in Ohio River sediments. Natural attenuation of PCBs was negligible in sediments, which was likely attributed to low temperature conditions during most of the year, as well as low amounts of available nitrogen, phosphorus, and organic carbon. -

Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis
International Journal of Molecular Sciences Review Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis David Johane Machate 1, Priscila Silva Figueiredo 2 , Gabriela Marcelino 2 , Rita de Cássia Avellaneda Guimarães 2,*, Priscila Aiko Hiane 2 , Danielle Bogo 2, Verônica Assalin Zorgetto Pinheiro 2, Lincoln Carlos Silva de Oliveira 3 and Arnildo Pott 1 1 Graduate Program in Biotechnology and Biodiversity in the Central-West Region of Brazil, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; [email protected] (D.J.M.); [email protected] (A.P.) 2 Graduate Program in Health and Development in the Central-West Region of Brazil, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; pri.fi[email protected] (P.S.F.); [email protected] (G.M.); [email protected] (P.A.H.); [email protected] (D.B.); [email protected] (V.A.Z.P.) 3 Chemistry Institute, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-67-3345-7416 Received: 9 March 2020; Accepted: 27 March 2020; Published: 8 June 2020 Abstract: Long-term high-fat dietary intake plays a crucial role in the composition of gut microbiota in animal models and human subjects, which affect directly short-chain fatty acid (SCFA) production and host health. This review aims to highlight the interplay of fatty acid (FA) intake and gut microbiota composition and its interaction with hosts in health promotion and obesity prevention and its related metabolic dysbiosis. -

Effect of Vertical Flow Exchange on Microbial Community Dis- Tributions in Hyporheic Zones
Article 1 by Heejung Kim and Kang-Kun Lee* Effect of vertical flow exchange on microbial community dis- tributions in hyporheic zones School of Earth and Environmental Sciences, Seoul National University, Seoul 08826, Republic of Korea; *Corresponding author, E-mail: [email protected] (Received: November 2, 2018; Revised accepted: January 6, 2019) https://doi.org/10.18814/epiiugs/2019/019001 The effect of the vertical flow direction of hyporheic flux advance of hydrodynamic modeling has improved research of hydro- on the bacterial community is examined. Vertical velocity logical exchange processes at the hyporheic zone (Cardenas and Wil- change of the hyporheic zone was examined by installing son, 2007; Fleckenstein et al., 2010; Endreny et al., 2011). Also, this a piezometer on the site, and a total of 20,242 reads were zone has plentiful micro-organisms. The hyporheic zone constituents analyzed using a pyrosequencing assay to investigate the a dynamic hotspot (ecotone) where groundwater and surface water diversity of bacterial communities. Proteobacteria (55.1%) mix (Smith et al., 2008). were dominant in the hyporheic zone, and Bacteroidetes This area constitutes a flow path along which surface water down wells into the streambed sediment and groundwater up wells in the (16.5%), Actinobacteria (7.1%) and other bacteria phylum stream, travels for some distance before eventually mixing with (Firmicutes, Cyanobacteria, Chloroflexi, Planctomycetesm groundwater returns to the stream channel (Hassan et al., 2015). Sur- and unclassified phylum OD1) were identified. Also, the face water enters the hyporheic zone when the vertical hydraulic head hyporheic zone was divided into 3 points – down welling of surface water is greater than the groundwater (down welling). -

Comparison of the Fecal Microbiota of Horses with Intestinal Disease and Their Healthy Counterparts
veterinary sciences Article Comparison of the Fecal Microbiota of Horses with Intestinal Disease and Their Healthy Counterparts Taemook Park 1,2, Heetae Cheong 3, Jungho Yoon 1, Ahram Kim 1, Youngmin Yun 2,* and Tatsuya Unno 4,5,* 1 Equine Clinic, Jeju Stud Farm, Korea Racing Authority, Jeju 63346, Korea; [email protected] (T.P.); [email protected] (J.Y.); [email protected] (A.K.) 2 College of Veterinary Medicine and Veterinary Medical Research Institute, Jeju National University, Jeju 63243, Korea 3 College of Veterinary Medicine and Institute of Veterinary Science, Kangwon National University, Chuncheon 24341, Korea; [email protected] 4 Faculty of Biotechnology, School of Life Sciences, SARI, Jeju 63243, Korea 5 Subtropical/Tropical Organism Gene Bank, Jeju National University, Jeju 63243, Korea * Correspondence: [email protected] (Y.Y.); [email protected] (T.U.); Tel.: +82-64-754-3376 (Y.Y.); +82-64-754-3354 (T.U.) Abstract: (1) Background: The intestinal microbiota plays an essential role in maintaining the host’s health. Dysbiosis of the equine hindgut microbiota can alter the fermentation patterns and cause metabolic disorders. (2) Methods: This study compared the fecal microbiota composition of horses with intestinal disease and their healthy counterparts living in Korea using 16S rRNA sequencing from fecal samples. A total of 52 fecal samples were collected and divided into three groups: horses with large intestinal disease (n = 20), horses with small intestinal disease (n = 8), and healthy horses (n = 24). (3) Results: Horses with intestinal diseases had fewer species and a less diverse bacterial population than healthy horses. -

Table S4. Phylogenetic Distribution of Bacterial and Archaea Genomes in Groups A, B, C, D, and X
Table S4. Phylogenetic distribution of bacterial and archaea genomes in groups A, B, C, D, and X. Group A a: Total number of genomes in the taxon b: Number of group A genomes in the taxon c: Percentage of group A genomes in the taxon a b c cellular organisms 5007 2974 59.4 |__ Bacteria 4769 2935 61.5 | |__ Proteobacteria 1854 1570 84.7 | | |__ Gammaproteobacteria 711 631 88.7 | | | |__ Enterobacterales 112 97 86.6 | | | | |__ Enterobacteriaceae 41 32 78.0 | | | | | |__ unclassified Enterobacteriaceae 13 7 53.8 | | | | |__ Erwiniaceae 30 28 93.3 | | | | | |__ Erwinia 10 10 100.0 | | | | | |__ Buchnera 8 8 100.0 | | | | | | |__ Buchnera aphidicola 8 8 100.0 | | | | | |__ Pantoea 8 8 100.0 | | | | |__ Yersiniaceae 14 14 100.0 | | | | | |__ Serratia 8 8 100.0 | | | | |__ Morganellaceae 13 10 76.9 | | | | |__ Pectobacteriaceae 8 8 100.0 | | | |__ Alteromonadales 94 94 100.0 | | | | |__ Alteromonadaceae 34 34 100.0 | | | | | |__ Marinobacter 12 12 100.0 | | | | |__ Shewanellaceae 17 17 100.0 | | | | | |__ Shewanella 17 17 100.0 | | | | |__ Pseudoalteromonadaceae 16 16 100.0 | | | | | |__ Pseudoalteromonas 15 15 100.0 | | | | |__ Idiomarinaceae 9 9 100.0 | | | | | |__ Idiomarina 9 9 100.0 | | | | |__ Colwelliaceae 6 6 100.0 | | | |__ Pseudomonadales 81 81 100.0 | | | | |__ Moraxellaceae 41 41 100.0 | | | | | |__ Acinetobacter 25 25 100.0 | | | | | |__ Psychrobacter 8 8 100.0 | | | | | |__ Moraxella 6 6 100.0 | | | | |__ Pseudomonadaceae 40 40 100.0 | | | | | |__ Pseudomonas 38 38 100.0 | | | |__ Oceanospirillales 73 72 98.6 | | | | |__ Oceanospirillaceae -

Role of Actinobacteria and Coriobacteriia in the Antidepressant Effects of Ketamine in an Inflammation Model of Depression
Pharmacology, Biochemistry and Behavior 176 (2019) 93–100 Contents lists available at ScienceDirect Pharmacology, Biochemistry and Behavior journal homepage: www.elsevier.com/locate/pharmbiochembeh Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression T Niannian Huanga,1, Dongyu Huaa,1, Gaofeng Zhana, Shan Lia, Bin Zhub, Riyue Jiangb, Ling Yangb, ⁎ ⁎ Jiangjiang Bia, Hui Xua, Kenji Hashimotoc, Ailin Luoa, , Chun Yanga, a Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China b Department of Internal Medicine, The Third Affiliated Hospital of Soochow University, Changzhou 213003, China c Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba 260-8670, Japan ARTICLE INFO ABSTRACT Keywords: Ketamine, an N-methyl-D-aspartic acid receptor (NMDAR) antagonist, elicits rapid-acting and sustained anti- Ketamine depressant effects in treatment-resistant depressed patients. Accumulating evidence suggests that gut microbiota Depression via the gut-brain axis play a role in the pathogenesis of depression, thereby contributing to the antidepressant Lipopolysaccharide actions of certain compounds. Here we investigated the role of gut microbiota in the antidepressant effects of Gut microbiota ketamine in lipopolysaccharide (LPS)-induced inflammation model of depression. Ketamine (10 mg/kg) sig- nificantly attenuated the increased immobility time in forced swimming test (FST), which was associated with the improvements in α-diversity, consisting of Shannon, Simpson and Chao 1 indices. In addition to α-diversity, β-diversity, such as principal coordinates analysis (PCoA), and linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe), showed a differential profile after ketamine treatment. -

Exploring Salivary Microbiota in AIDS Patients with Different Periodontal Statuses Using 454 GS-FLX Titanium Pyrosequencing
ORIGINAL RESEARCH published: 02 July 2015 doi: 10.3389/fcimb.2015.00055 Exploring salivary microbiota in AIDS patients with different periodontal statuses using 454 GS-FLX Titanium pyrosequencing Fang Zhang 1 †, Shenghua He 2 †, Jieqi Jin 1, Guangyan Dong 1 and Hongkun Wu 3* 1 State Key Laboratory of Oral Diseases, West China College of Stomatology, Sichuan University, Chengdu, China, 2 Public Health Clinical Center of Chengdu, Chengdu, China, 3 Department of Geriatric Dentistry, West China College of Stomatology, Sichuan University, Chengdu, China Patients with acquired immunodeficiency syndrome (AIDS) are at high risk of opportunistic infections. Oral manifestations have been associated with the level of immunosuppression, these include periodontal diseases, and understanding the microbial populations in the oral cavity is crucial for clinical management. The aim of this study was to examine the salivary bacterial diversity in patients newly admitted to the AIDS ward of the Public Health Clinical Center (China). Saliva samples were Edited by: Saleh A. Naser, collected from 15 patients with AIDS who were randomly recruited between December University of Central Florida, USA 2013 and March 2014. Extracted DNA was used as template to amplify bacterial Reviewed by: 16S rRNA. Sequencing of the amplicon library was performed using a 454 GS-FLX J. Christopher Fenno, University of Michigan, USA Titanium sequencing platform. Reads were optimized and clustered into operational Nick Stephen Jakubovics, taxonomic units for further analysis. A total of 10 bacterial phyla (106 genera) were Newcastle University, UK detected. Firmicutes, Bacteroidetes, and Proteobacteria were preponderant in the *Correspondence: salivary microbiota in AIDS patients. The pathogen, Capnocytophaga sp., and others Hongkun Wu, Department of Geriatric Dentistry, not considered pathogenic such as Neisseria elongata, Streptococcus mitis, and West China College of Stomatology, Mycoplasma salivarium but which may be opportunistic infective agents were detected. -

1 1 Children Developing Celiac Disease Have a Distinct And
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.29.971242; this version posted March 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 2 Children Developing Celiac Disease Have a Distinct and Proinflammatory Gut 3 Microbiota in the First 5 Years of Life 4 5 Qian Huang1, Yi Yang2, Vladimir Tolstikov3, Michael A. Kiebish3, Jonas F 6 Ludvigsson4,5, Noah W. Palm2, Johnny Ludvigsson6, Emrah Altindis1 7 8 9 Affiliations: 10 1 Boston College Biology Department, Chestnut Hill, MA 02467, USA 11 2 Department of Immunobiology, Yale University School of Medicine, New Haven, CT 12 06510, USA. 13 14 3 BERG, LLC, Framingham, MA, USA. 15 16 4 Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, 17 Stockholm, Sweden 18 19 5 Department of Paediatrics, Örebro University Hospital, Sweden 20 6 Crown Princess Victoria's Children's Hospital, Region Östergötland, Division of 21 Pediatrics, Linköping University, Linköping, SE 58185, Sweden. 22 23 24 25 26 27 Correspondence to: Emrah Altindis, Boston College Biology Department, Higgins Hall, 28 140 Commonwealth Avenue Chestnut Hill, MA 02467. E-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.29.971242; this version posted March 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 29 ABSTRACT 30 Objective: Celiac disease (CD) is an immune-mediated disease characterized by small intestinal 31 inflammation. -

Effect of Dietary Fat on the Metabolism of Energy and Nitrogen, Serum
bioRxiv preprint doi: https://doi.org/10.1101/438929; this version posted October 9, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Effect of Dietary Fat on the Metabolism of Energy and 2 Nitrogen, Serum Parameters, Rumen Fermentation, 3 and Microbiota in twin Hu Male Lambs 4 Wenjuan Lia, Hui Taoa, Naifeng Zhanga, Tao Maa, Kaidong Dengb, Biao Xiea, Peng Jiaa, Qiyu 5 Diaoa* 6 aFeed Research Institute, Key Laboratory of Feed Biotechnology of the Ministry of 7 Agriculture, Chinese Academy of Agricultural Sciences, Beijing 100081, China 8 bCollege of Animal Science, Jinling Institute of Technology, Nanjing, Jiangsu, China 9 *Corresponding author 10 E-mail:[email protected] 11 Abstract 12 Background: Fat is the main substance that provides energy to animals. However, 13 the use of fat in twin Hu lambs has not been investigated. Thirty pairs of male twin 14 lambs were examined to investigate the effects of dietary fat on the metabolism of 15 energy and nitrogen, ruminal fermentation, and microbial communities. The twins are 16 randomly allotted to two groups (high fat: HF, normal fat: NF). Two diets of equal 17 protein and different fat levels. The metabolism test was made at 50-60 days of age. 18 Nine pairs of twin lambs are slaughtered randomly, and the rumen fluid is collected at 19 60 days of age. 20 Results: The initial body weight (BW) in the HF group did not differ from that of NF 21 group (P > 0.05), but the final BW was tended to higher than that of NF group (0.05 < 22 P < 0.1). -
Supplementary Data TMAO Microbiome R1
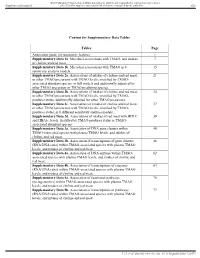
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Content for Supplementary Data Tables Tables Page Annotation guide for taxonomic features. 1 Supplementary Data 1a. Microbial associations with TMAO, and intakes 7 of choline and red meat. Supplementary Data 1b. Microbial associations with TMAO in 8 15 sensitivity analysis models. Supplementary Data 2a. Associations of intakes of choline and red meat, 21 or other TMAO precursors with TMAO levels, stratified by TMAO- associated abundant species, in full models and additionally adjusted for other TMAO precursors or TMAO predicting species. Supplementary Data 2b. Associations of intakes of choline and red meat, 34 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, additionally adjusted for other TMAO precursors. Supplementary Data 2c. Associations of intakes of choline and red meat, 37 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, in 6 different sensitivity analysis models. Supplementary Data 2d. Associations of intakes of red meat with HDLC 39 and HBA1c levels, stratified by TMAO-producer status or TMAO- associated abundant species. Supplementary Data 3a. Association of DNA gene clusters within 40 TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 3b. Association of transcriptions of gene clusters 46 (RNA/DNA ratio) within TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 4a. Association of DNA enzyme within TMAO- 62 associated species with plasma TMAO levels, and intakes of choline and red meat. -

Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea—A Systematic Review of the Literature
microorganisms Review Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea—A Systematic Review of the Literature Klaudia Tutka, Magdalena Zychowska˙ and Adam Reich * Department of Dermatology, Institute of Medical Sciences, Medical College of Rzeszow University, 35-055 Rzeszow, Poland; [email protected] (K.T.); [email protected] (M.Z.)˙ * Correspondence: [email protected]; Tel.: +48-605076722 Received: 30 August 2020; Accepted: 6 November 2020; Published: 8 November 2020 Abstract: Rosacea is a chronic inflammatory skin disorder of a not fully understood pathophysiology. Microbial factors, although not precisely characterized, are speculated to contribute to the development of the condition. The aim of the current review was to summarize the rosacea-associated alterations in the skin, blood, and gut microbiome, investigated using culture-independent, metagenomic techniques. A systematic review of the PubMed, Web of Science, and Scopus databases was performed, according to PRISMA (preferred reporting items for systematic review and meta-analyses) guidelines. Nine out of 185 papers were eligible for analysis. Skin microbiome was investigated in six studies, and in a total number of 115 rosacea patients. Blood microbiome was the subject of one piece of research, conducted in 10 patients with rosacea, and gut microbiome was studied in two papers, and in a total of 23 rosacea subjects. Although all of the studies showed significant alterations in the composition of the skin, blood, or gut microbiome in rosacea, the results were highly inconsistent, or even, in some cases, contradictory. Major limitations included the low number of participants, and different study populations (mainly Asians). Further studies are needed in order to reliably analyze the composition of microbiota in rosacea, and the potential application of microbiome modifications for the treatment of this dermatosis. -

Bacterial Diversity and Functional Analysis of Severe Early Childhood
www.nature.com/scientificreports OPEN Bacterial diversity and functional analysis of severe early childhood caries and recurrence in India Balakrishnan Kalpana1,3, Puniethaa Prabhu3, Ashaq Hussain Bhat3, Arunsaikiran Senthilkumar3, Raj Pranap Arun1, Sharath Asokan4, Sachin S. Gunthe2 & Rama S. Verma1,5* Dental caries is the most prevalent oral disease afecting nearly 70% of children in India and elsewhere. Micro-ecological niche based acidifcation due to dysbiosis in oral microbiome are crucial for caries onset and progression. Here we report the tooth bacteriome diversity compared in Indian children with caries free (CF), severe early childhood caries (SC) and recurrent caries (RC). High quality V3–V4 amplicon sequencing revealed that SC exhibited high bacterial diversity with unique combination and interrelationship. Gracillibacteria_GN02 and TM7 were unique in CF and SC respectively, while Bacteroidetes, Fusobacteria were signifcantly high in RC. Interestingly, we found Streptococcus oralis subsp. tigurinus clade 071 in all groups with signifcant abundance in SC and RC. Positive correlation between low and high abundant bacteria as well as with TCS, PTS and ABC transporters were seen from co-occurrence network analysis. This could lead to persistence of SC niche resulting in RC. Comparative in vitro assessment of bioflm formation showed that the standard culture of S. oralis and its phylogenetically similar clinical isolates showed profound bioflm formation and augmented the growth and enhanced bioflm formation in S. mutans in both dual and multispecies cultures. Interaction among more than 700 species of microbiota under diferent micro-ecological niches of the human oral cavity1,2 acts as a primary defense against various pathogens. Tis has been observed to play a signifcant role in child’s oral and general health.