Cruro-Pedal Structure of the Paulchoffatiid Rugosodon Eurasiaticus and Evolution of the Multituberculate Ankle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reptile-Like Physiology in Early Jurassic Stem-Mammals
bioRxiv preprint doi: https://doi.org/10.1101/785360; this version posted October 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title: Reptile-like physiology in Early Jurassic stem-mammals Authors: Elis Newham1*, Pamela G. Gill2,3*, Philippa Brewer3, Michael J. Benton2, Vincent Fernandez4,5, Neil J. Gostling6, David Haberthür7, Jukka Jernvall8, Tuomas Kankanpää9, Aki 5 Kallonen10, Charles Navarro2, Alexandra Pacureanu5, Berit Zeller-Plumhoff11, Kelly Richards12, Kate Robson-Brown13, Philipp Schneider14, Heikki Suhonen10, Paul Tafforeau5, Katherine Williams14, & Ian J. Corfe8*. Affiliations: 10 1School of Physiology, Pharmacology & Neuroscience, University of Bristol, Bristol, UK. 2School of Earth Sciences, University of Bristol, Bristol, UK. 3Earth Science Department, The Natural History Museum, London, UK. 4Core Research Laboratories, The Natural History Museum, London, UK. 5European Synchrotron Radiation Facility, Grenoble, France. 15 6School of Biological Sciences, University of Southampton, Southampton, UK. 7Institute of Anatomy, University of Bern, Bern, Switzerland. 8Institute of Biotechnology, University of Helsinki, Helsinki, Finland. 9Department of Agricultural Sciences, University of Helsinki, Helsinki, Finland. 10Department of Physics, University of Helsinki, Helsinki, Finland. 20 11Helmholtz-Zentrum Geesthacht, Zentrum für Material-und Küstenforschung GmbH Germany. 12Oxford University Museum of Natural History, Oxford, OX1 3PW, UK. 1 bioRxiv preprint doi: https://doi.org/10.1101/785360; this version posted October 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 13Department of Anthropology and Archaeology, University of Bristol, Bristol, UK. 14Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, UK. -

Micheli Stefanello
UNIVERSIDADE FEDERAL DE SANTA MARIA CENTRO DE CIÊNCIAS NATURAIS E EXATAS PROGRAMA DE PÓS-GRADUAÇÃO EM BIODIVERSIDADE ANIMAL Micheli Stefanello DESCRIÇÃO E FILOGENIA DE UM NOVO ESPÉCIME DE CINODONTE PROBAINOGNÁTIO DO TRIÁSSICO SUL-BRASILEIRO Santa Maria, RS 2018 Micheli Stefanello DESCRIÇÃO E FILOGENIA DE UM NOVO ESPÉCIME DE CINODONTE PROBAINOGNÁTIO DO TRIÁSSICO SUL-BRASILEIRO Dissertação apresentada ao Curso de Mestrado do Programa de Pós-Graduação em Biodiversidade Animal, Área de Concentração em Sistemática e Biologia Evolutiva, da Universidade Federal de Santa Maria (UFSM, RS), como requisito parcial para obtenção do grau de Mestre em Ciências Biológicas – Área Biodiversidade Animal. Orientador: Prof. Dr. Sérgio Dias da Silva Santa Maria, RS 2018 Micheli Stefanello DESCRIÇÃO E FILOGENIA DE UM NOVO ESPÉCIME DE CINODONTE PROBAINOGNÁTIO DO TRIÁSSICO SUL-BRASILEIRO Dissertação apresentada ao Curso de Mestrado do Programa de Pós-Graduação em Biodiversidade Animal, Área de Concentração em Sistemática e Biologia Evolutiva, da Universidade Federal de Santa Maria (UFSM, RS), como requisito parcial para obtenção do grau de Mestre em Ciências Biológicas – Área Biodiversidade Animal. Aprovada em 28 de fevereiro de 2018: Santa Maria, RS 2018 AGRADECIMENTOS Ao meu orientador, Dr. Sérgio Dias da Silva, pela orientação, por todo o tempo despendido ao longo desse mestrado e por possibilitar meu aprimoramento na área a qual tenho apreço. Aos colegas do Centro de Apoio à Pesquisa Paleontológica da Quarta Colônia da Universidade Federal de Santa Maria (CAPPA/UFSM) e do Laboratório de Paleobiodiversidade Triássica, dessa mesma instituição, pela convivência e por terem ajudado-me de diferentes formas ao longo do mestrado. Em especial, ao colega Rodrigo Temp Müller, pela coleta do espécime (objeto de estudo dessa dissertação), por toda a ajuda com o TNT e com as figuras, e por auxiliar-me de inúmeras formas. -

Miocene Mammal Reveals a Mesozoic Ghost Lineage on Insular New Zealand, Southwest Pacific
Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific Trevor H. Worthy*†, Alan J. D. Tennyson‡, Michael Archer§, Anne M. Musser¶, Suzanne J. Hand§, Craig Jonesʈ, Barry J. Douglas**, James A. McNamara††, and Robin M. D. Beck§ *School of Earth and Environmental Sciences, Darling Building DP 418, Adelaide University, North Terrace, Adelaide 5005, South Australia, Australia; ‡Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington 6015, New Zealand; §School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia; ¶Australian Museum, 6-8 College Street, Sydney, New South Wales 2010, Australia; ʈInstitute of Geological and Nuclear Sciences, P.O. Box 30368, Lower Hutt 5040, New Zealand; **Douglas Geological Consultants, 14 Jubilee Street, Dunedin 9011, New Zealand; and ††South Australian Museum, Adelaide, South Australia 5000, Australia Edited by James P. Kennett, University of California, Santa Barbara, CA, and approved October 11, 2006 (sent for review July 8, 2006) New Zealand (NZ) has long been upheld as the archetypical Ma) dinosaur material (13) and isolated moa bones from marine example of a land where the biota evolved without nonvolant sediments up to 2.5 Ma (1, 14), the terrestrial record older than terrestrial mammals. Their absence before human arrival is mys- 1 Ma is extremely limited. Until now, there has been no direct terious, because NZ was still attached to East Antarctica in the Early evidence for the pre-Pleistocene presence in NZ of any of its Cretaceous when a variety of terrestrial mammals occupied the endemic vertebrate lineages, particularly any group of terrestrial adjacent Australian portion of Gondwana. -

Mammalian Origins Major Groups of Synapsida
Mammalogy 4764 9/16/2009 Chapter 4 “Reptile” Mammalian Origins Early mammal Carnivore Amniota Herbivore Feldhamer Table 4.1 Savage and Long 1986 Major Fig. 3.2, Vaughn, Fig. 4.1, Feldhamer groups Mammalian Origins of Dimetrodon Overview Synapsida Synapsids Pelycosaurs and Therapsids First Mammals Mesozoic Era appear Feldhamer Fig. 4.2 Cenozoic Era radiate Savage and Long 1986 Pelycosaur Skull, jaw musculature, and teeth Cynodontia -- Advanced, predaceous therapsids Pelycosaur Scymnognathus Cynognathus Therapsid Early Cynodont Derived Therapsid/Mammal Primitive Late Cynodont Fig. 3.2, Vaughn Thrinaxodon Fig 4.3 & 4, Feldhamer 1 Mammalogy 4764 9/16/2009 Skeletal transition Extinction of Cynodonts Possibly competition from dinosaurs Pelycosaur Early Cynodonts were dog-size, last surviving were squirrel sized Fig. 4.15 Mammals that survived while Cynodonts went extinct (contemporary) were mouse-sized. Cynodont Thrinaxodon Modern Mammal Fig. 3.5, Vaughn Fig. 4.16c, Early Cynodont Early mammals Changes in land masses Feldhamer 4.11 200 - 250 million years ago Derived characters: Dentary/squamosal jaw articulation Diphyodont dentition 200 MYA 180 MYA Mammary glands Secondary palate Early Mid- Viviparity (loss of eggshell) When? Jurassic Jurassic 65 MYA 135 MYA Early Early Cretaceous Cenozoic Feldhamer 4.5, 4.9 Skull and teeth of mammals 2 Mammalogy 4764 9/16/2009 Teeth and Dentition of Mammals Teeth Heterodont teeth with different functions Differentiated on the basis of function, resulting in increased One of the major keys efficiency acquiring and digesting food. to success of mammals Teeth occur in 3 bones of skull: Teeth of mammals are premaxilla, maxilla, dentary extremely variable with different diets -- more than other taxa Feldhamer et al. -

Femur of a Morganucodontid Mammal from the Middle Jurassic of Central Russia
Femur of a morganucodontid mammal from the Middle Jurassic of Central Russia PETR P. GAMBARYAN and ALEXANDER 0.AVERIANOV Gambaryan, P.P. & Averianov, A.O. 2001. Femur of a morganucodontid mammal from the Middle Jurassic of Central Russia. -Acta Palaeontologica Polonica 46,1,99-112. We describe a nearly complete mammalian femur from the Middle Jurassic (upper Bathonian) from Peski quarry, situated some 100 km south east of Moscow, central Rus- sia. It is similar to the femora of Morganucodontidae in having a globular femoral head, separated from the greater trochanter and reflected dorsally, fovea capitis present, both trochanters triangular and located on the same plane, distal end flat, mediolaterally expanded, and somewhat bent ventrally, and in the shape and proportions of distal condyles. It is referred to as Morganucodontidae gen. et sp. indet. It is the first representa- tive of this group of mammals in Eastern Europe from the third Mesozoic mammal local- ity discovered in Russia. Exquisite preservation of the bone surface allowed us to recon- struct partial hind limb musculature. We reconstruct m. iliopsoas as inserting on the ridge, which starts at the lesser trochanter and extends along the medial femoral margin for more than half of the femur length. On this basis we conclude that the mode of loco- motion of the Peski morganucodontid was similar to that of modern echidnas. During the propulsive phase the femur did not retract and the step elongation was provided by pronation of the femur. Key words : Mammalia, Morganucodontidae, femur, anatomy, locomotion, Jurassic, Russia. Petr P. Gambaryan [[email protected]] and Alexander 0. -

Paleontological Discoveries in the Chorrillo Formation (Upper Campanian-Lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina
Rev. Mus. Argentino Cienc. Nat., n.s. 21(2): 217-293, 2019 ISSN 1514-5158 (impresa) ISSN 1853-0400 (en línea) Paleontological discoveries in the Chorrillo Formation (upper Campanian-lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina Fernando. E. NOVAS1,2, Federico. L. AGNOLIN1,2,3, Sebastián ROZADILLA1,2, Alexis M. ARANCIAGA-ROLANDO1,2, Federico BRISSON-EGLI1,2, Matias J. MOTTA1,2, Mauricio CERRONI1,2, Martín D. EZCURRA2,5, Agustín G. MARTINELLI2,5, Julia S. D´ANGELO1,2, Gerardo ALVAREZ-HERRERA1, Adriel R. GENTIL1,2, Sergio BOGAN3, Nicolás R. CHIMENTO1,2, Jordi A. GARCÍA-MARSÀ1,2, Gastón LO COCO1,2, Sergio E. MIQUEL2,4, Fátima F. BRITO4, Ezequiel I. VERA2,6, 7, Valeria S. PEREZ LOINAZE2,6 , Mariela S. FERNÁNDEZ8 & Leonardo SALGADO2,9 1 Laboratorio de Anatomía Comparada y Evolución de los Vertebrados. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina - fernovas@yahoo. com.ar. 2 Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina. 3 Fundación de Historia Natural “Felix de Azara”, Universidad Maimonides, Hidalgo 775, C1405BDB Buenos Aires, Argentina. 4 Laboratorio de Malacología terrestre. División Invertebrados Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 5 Sección Paleontología de Vertebrados. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 6 División Paleobotánica. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 7 Área de Paleontología. Departamento de Geología, Universidad de Buenos Aires, Pabellón 2, Ciudad Universitaria (C1428EGA) Buenos Aires, Argentina. 8 Instituto de Investigaciones en Biodiversidad y Medioambiente (CONICET-INIBIOMA), Quintral 1250, 8400 San Carlos de Bariloche, Río Negro, Argentina. -

A New Mammaliaform from the Early Jurassic and Evolution Of
R EPORTS tary trough with a shelflike dorsal medial ridge, and all other nonmammalian mamma- A New Mammaliaform from the liaforms have a medial concavity on the man- dibular angle (8–14, 23), as in nonmamma- Early Jurassic and Evolution of liaform cynodonts (9, 14, 24–27). The post- dentary trough and the medial concavity on Mammalian Characteristics the mandibular angle respectively accommo- dated the prearticular/surangular and the re- Zhe-Xi Luo,1* Alfred W. Crompton,2 Ai-Lin Sun3 flected lamina of the angular (9, 25–27) that are the homologs to the mammalian middle A fossil from the Early Jurassic (Sinemurian, ϳ195 million years ago) represents ear bones (9, 14, 16–21, 23, 26). The absence a new lineage of mammaliaforms, the extinct groups more closely related to of these structures indicates that the postden- the living mammals than to nonmammaliaform cynodonts. It has an enlarged tary bones (“middle ear ossicles”) must have cranial cavity, but no postdentary trough on the mandible, indicating separation been separated from the mandible (Fig. 3). of the middle ear bones from the mandible. This extends the earliest record of Hadrocodium lacks the primitive meckelian these crucial mammalian features by some 45 million years and suggests that sulcus of the mandible typical of all nonmam- separation of the middle ear bones from the mandible and the expanded brain maliaform cynodonts (24–27), stem groups vault could be correlated. It shows that several key mammalian evolutionary of mammaliaforms (8, 9, 14, 23, 26, 27), innovations in the ear region, the temporomandibular joint, and the brain vault triconodontids (28, 29), and nontribosphenic evolved incrementally through mammaliaform evolution and long before the therian mammals (30). -

Mesozoic: the Dark Age for Mammals!
Ed’s Simplified History of the Mammals Note progression from Pelycosaurs (1) to Therapsids and Cynodonts (2) in Triassic. Stem mammals appeared in Late Triassic and Early Jurassic (3). Relationships among the Middle Jurassic forms (4) are controversial (see handout). Therian clade, identified by the tribosphenic molar (5), emerged at the end of the Jurassic, Early Cretaceous. A slightly more detailed version… in case you like something that looks more slick From Pough et al. 2009. Vertebrate Life, 8th ed. Pelycosaurs Dominated the late Permian, gave rise to therapsids Therapsids Rapid radiation in late Permian, around 270 MYA Still “mammal-like reptiles” The mass extinction at the end of the Permian was the greatest loss of diversity ever with >80% of all known genera and about 90% of all species going extinct, both terrestrial and marine. Cynodonts Late Permian to mid Triassic Last remaining group of therapsids, survived mass extinction at the end of the Permian. Persisted well Only 1 lineage of into Triassic and developed cynodonts survived many features associated through the late Triassic, with mammals. and this group became ancestors of mammals. Mesozoic: the Dark Age for Mammals! multituberculate Morganucodon, one of the earliest mammals (What else was happening in the Late Triassic and Jurassic Hadrocodium that may have contributed to mammals becoming small and Most were very small with nocturnal?) conservative morphology ...but new fossil finds indicate more diversity than we thought Repenomanus Still, largest known mammal during Mesozic Most were shrew to is no larger than a mouse sized, for 125 woodchuck million years! Some Mesozoic events and mammals you should know 1. -

Gilles Cuny, a Late Triassic Cynodont from Holwell Quarries
ORYCTOS, Vol. 5 : 69 - 73, Décembre 2004 A LATE TRIASSIC CYNODONT FROM HOLWELL QUARRIES (SOMERSET, ENGLAND) Gilles CUNY Geological Museum, University of Copenhagen, Øster Voldgade 5-7, 1350 Copenhagen K, Denmark Abstract : The presence of the dromatheriid cynodont Pseudotriconodon wildi is reported from the Rhaetian of Great Britain in the fissure deposits at Holwell Quarries. The British Rhaetic cynodont fauna, although consisting only of one dromatheriid tooth, one tooth of Tricuspes, and one tritylodontid fragmentary jaw, is similar to that found in the Germanic Realm. It appears to be the remnant of a fauna that covered all of Western Europe before the fragmentation of its habitat due to the Rhaetian transgression. Keywords : Cynodontia, Triassic, Rhaetian, Great Britain, Europe. Un cynodonte dans le Trias supérieur des carrières d’Holwell (Somerset, Angleterre) Résumé : La présence du cynodonte Pseudotriconodon wildi est signalée dans des remplissages de fissures rhétiens des carrières d’Holwell, en Angleterre. La faune de cynodontes du Rhétien d’Angleterre, bien que connue unique- ment d’après une dent de Dromatheriidés, une dent de Tricuspes, et un fragment de mâchoire de Tritylodontidés, est similaire à celle trouvée à la même époque en Europe continentale dans le bassin germanique. Il s’agit probablement des restes d’une faune plus ancienne qui couvrait l’ensemble de l’Europe avant que celle-ci ne soit fragmentée en plusieurs îles par la transgression rhétienne. Mots clés : Cynodontia, Trias, Rhétien, Europe, Grande-Bretagne. INTRODUCTION Moore (1859, 1861, 1862, 1864, 1867) was the first to study these fissures, set in quarries that are now The fissure fillings at Holwell quarries have closed. -

Parental Care Or Opportunism in South African Triassic Cynodonts?
Parental care or opportunism in South African AUTHOR: Triassic cynodonts? Julien Benoit1 AFFILIATION: In a paper published in Nature in 2018, Hoffman and Rowe1 describe the discovery of an adult tritylodontid cynodont, 1Evolutionary Studies Institute, Kayentatherium, from the Jurassic of the Kayenta Formation (Arizona, USA), accompanied by at least 38 perinatal School of Geosciences, University juveniles, all at the same very early stage of development. Such a high number of juveniles in one clutch is found of the Witwatersrand, Johannesburg, only in a handful of oviparous reptiles, and never in viviparous or ovoviviparous species1, suggesting that these South Africa cynodonts laid eggs. As tritylodontids are amongst the closest relatives to Mammaliaformes, and sometimes even reconstructed as their sister clade2, this textbook changing discovery implies that all non-mammaliaform CORRESPONDENCE TO: synapsids had an essentially reptilian-like reproductive biology1. Julien Benoit Hoffman and Rowe’s1 discovery forces a reappraisal of the interpretation of the rich fossil record of parental care EMAIL: in South African non-mammaliaform cynodonts3. [email protected] First, the implications of Hoffman and Rowe’s1 discovery for the evolution of parental care and lactation need to be discussed. With at least 38 hatchlings to feed, it can be safely assumed that lactation was not the main source of HOW TO CITE: Benoit J. Parental care or nutrients in Kayentatherium neonates (nor, by extension, in all more basal non-mammaliaform synapsids). Instead, opportunism in South African perinatal juveniles of Kayentatherium already had functional teeth despite their early age, which (1) impedes Triassic cynodonts? S Afr J Sci. -
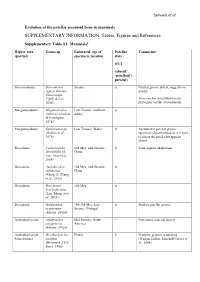
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

Origin and Relationships of the Ictidosauria to Non-Mammalian
Historical Biology An International Journal of Paleobiology ISSN: 0891-2963 (Print) 1029-2381 (Online) Journal homepage: http://www.tandfonline.com/loi/ghbi20 Origin and relationships of the Ictidosauria to non- mammalian cynodonts and mammals José F. Bonaparte & A. W. Crompton To cite this article: José F. Bonaparte & A. W. Crompton (2017): Origin and relationships of the Ictidosauria to non-mammalian cynodonts and mammals, Historical Biology, DOI: 10.1080/08912963.2017.1329911 To link to this article: http://dx.doi.org/10.1080/08912963.2017.1329911 Published online: 23 Jun 2017. Submit your article to this journal Article views: 53 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ghbi20 Download by: [Smithsonian Astrophysics Observatory] Date: 06 November 2017, At: 13:06 HISTORICAL BIOLOGY, 2017 https://doi.org/10.1080/08912963.2017.1329911 Origin and relationships of the Ictidosauria to non-mammalian cynodonts and mammals José F. Bonapartea and A. W. Cromptonb aMuseo Municipal de C. Naturales “C. Ameghino”, Mercedes, Argentina; bMuseum of Comparative Zoology, Harvard University, Cambridge, MA, USA ABSTRACT ARTICLE HISTORY Ictidosaurian genera are allocated to two families, Tritheledontidae and Therioherpetidae. This paper Received 19 December 2016 provides a diagnosis for Ictidosauria. The previously named family Brasilodontidae is shown to be a Accepted 30 April 2017 junior synonym of a family, Therioherpetidae. It is concluded that Ictidosauria originated from Late KEYWORDS Permian procynosuchid non-mammalian cynodonts rather than from Middle Triassic probainognathid Mammalian origins; non-mammalian cynodonts. The structure of the skull and jaws of a derived traversodontid Ischignathus Ictidosauria; Tritylodontia; sudamericanus from the early Late Triassic of Argentina supports an earlier view that tritylodontids are Brasilitherium more closely related to traversodontid than probainognathid non-mammalian cynodonts.