Evolution, Homology, and Development of Tetrapod Limb Muscles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On the Pelvic Girdle and Pin of Eusthenopteron. by Edwin S
PELVIC GIRDLE AND D'lN . OF EUSTHKNOPfERON. 311 On the Pelvic Girdle and Pin of Eusthenopteron. By Edwin S. Goodrich, M.A., Fellow of Mertoa College, Oxford. With Plate 16. 1 THROUGH the kindness of Mr. A. Smith Woodward, I have recently had the opportunity of looking through the fossil fish acquired by the British Museum since the Cata- logue was published. Amongst these was found a specimen of Eusthenopteron foordi, Whit., showing the endo- skeleton of the pelvic girdle and fin, of which I here give a description. The interest attaching to this fossil is con- siderable, since, of all the numerous extinct fish usually included in the group " Crossopterygii," it is the first and only one in which the parts of the skeleton of the pelvic girdle and its fin have been found complete and in their natural relations.2 The specimen (P. 6794) of which both the slab and the counterslab have been preserved, comes from the Upper Devonian of Canada. In it can be made out the skeleton of the pelvic girdle and fin of the right side, in a fairly com- plete and well-preserved condition, as represented in PI. 16, fig. 1, natural size. 1 To Mr. Smith Woodward I am also indebted for constant help when working in his Department. a The skeleton of the pelvic fin of Megalichthys has to some extent been made known by Cope, Miall, and Wellburn (2, 5, and 9), and the essential structure of that of Eusthenopteron has been briefly described by Traquair (7). VOL. 45, FART 2.—NEW SKKIES. -

New Permian Fauna from Tropical Gondwana
ARTICLE Received 18 Jun 2015 | Accepted 18 Sep 2015 | Published 5 Nov 2015 DOI: 10.1038/ncomms9676 OPEN New Permian fauna from tropical Gondwana Juan C. Cisneros1,2, Claudia Marsicano3, Kenneth D. Angielczyk4, Roger M. H. Smith5,6, Martha Richter7, Jo¨rg Fro¨bisch8,9, Christian F. Kammerer8 & Rudyard W. Sadleir4,10 Terrestrial vertebrates are first known to colonize high-latitude regions during the middle Permian (Guadalupian) about 270 million years ago, following the Pennsylvanian Gondwanan continental glaciation. However, despite over 150 years of study in these areas, the bio- geographic origins of these rich communities of land-dwelling vertebrates remain obscure. Here we report on a new early Permian continental tetrapod fauna from South America in tropical Western Gondwana that sheds new light on patterns of tetrapod distribution. Northeastern Brazil hosted an extensive lacustrine system inhabited by a unique community of temnospondyl amphibians and reptiles that considerably expand the known temporal and geographic ranges of key subgroups. Our findings demonstrate that tetrapod groups common in later Permian and Triassic temperate communities were already present in tropical Gondwana by the early Permian (Cisuralian). This new fauna constitutes a new biogeographic province with North American affinities and clearly demonstrates that tetrapod dispersal into Gondwana was already underway at the beginning of the Permian. 1 Centro de Cieˆncias da Natureza, Universidade Federal do Piauı´, 64049-550 Teresina, Brazil. 2 Programa de Po´s-Graduac¸a˜o em Geocieˆncias, Departamento de Geologia, Universidade Federal de Pernambuco, 50740-533 Recife, Brazil. 3 Departamento de Cs. Geologicas, FCEN, Universidad de Buenos Aires, IDEAN- CONICET, C1428EHA Ciudad Auto´noma de Buenos Aires, Argentina. -

Forelimb Position Affects Facultative Bipedal Locomotion in Lizards Chase T
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb185975. doi:10.1242/jeb.185975 RESEARCH ARTICLE Forelimb position affects facultative bipedal locomotion in lizards Chase T. Kinsey* and Lance D. McBrayer‡ ABSTRACT 2004; Clark and Higham, 2011; Tucker and McBrayer, 2012; Parker Recent work indicates that bipedal posture in lizards is advantageous and McBrayer, 2016). During predation events or social ’ during obstacle negotiation. However, little is known about how interactions, a terrestrial vertebrate s behavior, speed and stability bipedalism occurs beyond a lizard’s acceleratory threshold. traversing obstacles may impinge upon their survivorship and/or Furthermore, no study to date has examined the effects of forelimb fitness (Stiller and McBrayer, 2013; Schulte et al., 2004; Arnold, position on the body center of mass (BCoM) in the context of 1983; but see Garland and Losos, 1994). bipedalism. This study quantified the frequency of bipedalism when Stereotyped limb movement in quadrupedal locomotion, or gait, sprinting with versus without an obstacle at 0.8 m from the start of a has predictable footfalls across various speeds (Snyder, 1952, 1954, sprint. Forelimb positions were quantified during bipedal running at 1962; Irschick and Jayne, 1999; Farley and Ko, 1997). Some the start of a sprint and when crossing an obstacle. Two species with terrestrial lizards alter their gait and/or posture while sprinting contrasting body forms (and thus different BCoM) were studied (Schuett et al., 2009; for review, see Russell and Bels, 2001). (Sceloporus woodi and Aspidoscelis sexlineata) to assess potential Facultative bipedalism occurs in some quadrupeds when only the variation due to body plan and obstacle-crossing behavior. -

Stuttgarter Beiträge Zur Naturkunde
S^5 ( © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie) Herausgeber: Staatliches Museum für Naturkunde, Rosenstein 1, D-70191 Stuttgart Stuttgarter Beitr. Naturk. Ser. B Nr. 278 175 pp., 4pls., 54figs. Stuttgart, 30. 12. 1999 Comparative osteology oi Mastodonsaurus giganteus (Jaeger, 1828) from the Middle Triassic (Lettenkeuper: Longobardian) of Germany (Baden-Württemberg, Bayern, Thüringen) By Rainer R. Schoch, Stuttgart With 4 plates and 54 textfigures Abstract Mastodonsaurus giganteus, the most abundant and giant amphibian of the German Letten- keuper, is revised. The study is based on the excellently preserved and very rieh material which was excavated during road construction in 1977 near Kupferzeil, Northern Baden- Württemberg. It is shown that there exists only one diagnosable species of Mastodonsaurus, to which all Lettenkeuper material can be attributed. All finds from other horizons must be referred to as Mastodonsauridae gen. et sp. indet. because of their fragmentary Status. A sec- ond, definitely diagnostic genus of this family is Heptasaurus from the higher Middle and Upper Buntsandstein. Finally a diagnosis of the family Mastodonsauridae is provided. Ä detailed osteological description of Mastodonsaurus giganteus reveals numerous un- known or formerly inadequately understood features, yielding data on various hitherto poor- ly known regions of the skeleton. The sutures of the skull roof, which could be studied in de- tail, are significantly different from the schemes presented by previous authors. The endocra- nium and mandible are further points of particular interest. The palatoquadrate contributes a significant part to the formation of the endocranium by an extensive and complicated epi- pterygoid. -

A New Osteolepidid Fish From
Rea. West. Aust. MU8. 1985, 12(3): 361-377 ANew Osteolepidid Fish from the Upper Devonian Gogo Formation, Western Australia J.A. Long* Abstract A new osteolepidid crossopterygian, Gogonasus andrewsi gen. et sp. nov., is des cribed from a single fronto-ethmoidal shield and associated ethmosphenoid, from the Late Devonian (Frasnian) Gogo Formation, Western Australia. Gogonasus is is distinguished from other osteolepids by the shape and proportions of the fronto ethmoidal shield, absence of palatal fenestrae, well developed basipterygoid pro cesses and moderately broad parasphenoid. The family Osteolepididae is found to be paraphyletic, with Gogonasus being regarded as a plesiomorphic osteolepidid at a similar level of organisation to Thursius. Introduction Much has been published on the well-preserved Late Devonian fish fauna from the Gogo Formation, Western Australia, although to date all the papers describing fish have been on placoderms (Miles 1971; Miles and Dennis 1979; Dennis and Miles 1979-1983; Young 1984), palaeoniscoids (Gardiner 1973, 1984; Gardiner and Bartram 1977) or dipnoans (Miles 1977; Campbell and Barwick 1982a, 1982b, 1983, 1984a). This paper describes the only osteolepiform from the fauna (Gardiner and Miles 1975), a small snout with associated braincase, ANU 21885, housed in the Geology Department, Australian National University. The specimen, collected by the Australian National University on the 1967 Gogo Expedition, was prepared by Dr S.M. Andrews (Royal Scottish Museum) and later returned to the ANU. Onychodus is the only other crossopterygian in the fauna. In its proportions and palatal structure the new specimen provides some additional new points of the anatomy of osteolepiforms. Few Devonian crossopte rygians are known from Australia, and so the specimen is significant in having resemblances to typical Northern Hemisphere species. -

Development and the Evolvability of Human Limbs
Development and the evolvability of human limbs Nathan M. Younga,1, Günter P. Wagnerb, and Benedikt Hallgrímssonc aDepartment of Orthopaedic Surgery, University of California, San Francisco, CA 94110; bDepartment of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06405; and cDepartment of Cell Biology and Anatomy, University of Calgary, Calgary, AB, Canada T2N4N1 Edited* by David Pilbeam, Harvard University, Cambridge, MA, and approved December 29, 2009 (received for review October 14, 2009) The long legs and short arms of humans are distinctive for a fore- and hindlimb modules was reduced, leading to a higher primate, the result of selection acting in opposite directions on degree of variational independence of limb size and shape. Our each limb at different points in our evolutionary history. This model predicts that selection for independent function of the mosaic pattern challenges our understanding of the relationship limbs should lead to alterations to limb covariational structure. of development and evolvability because limbs are serially homol- Specifically, humans and apes would be predicted to exhibit ogous and genetic correlations should act as a significant con- decreased phenotypic correlations between fore- and hindlimb straint on their independent evolution. Here we test a compared to quadrupeds. If decreases in integration between developmental model of limb covariation in anthropoid primates fore- and hindlimb are a product of selection for more inde- and demonstrate that both humans and apes exhibit significantly pendent function of limbs, then the timing of any change in reduced integration between limbs when compared to quadrupe- integration has implications for the reconstruction of ancestral dal monkeys. -

Morphology, Phylogeny, and Evolution of Diadectidae (Cotylosauria: Diadectomorpha)
Morphology, Phylogeny, and Evolution of Diadectidae (Cotylosauria: Diadectomorpha) by Richard Kissel A thesis submitted in conformity with the requirements for the degree of doctor of philosophy Graduate Department of Ecology & Evolutionary Biology University of Toronto © Copyright by Richard Kissel 2010 Morphology, Phylogeny, and Evolution of Diadectidae (Cotylosauria: Diadectomorpha) Richard Kissel Doctor of Philosophy Graduate Department of Ecology & Evolutionary Biology University of Toronto 2010 Abstract Based on dental, cranial, and postcranial anatomy, members of the Permo-Carboniferous clade Diadectidae are generally regarded as the earliest tetrapods capable of processing high-fiber plant material; presented here is a review of diadectid morphology, phylogeny, taxonomy, and paleozoogeography. Phylogenetic analyses support the monophyly of Diadectidae within Diadectomorpha, the sister-group to Amniota, with Limnoscelis as the sister-taxon to Tseajaia + Diadectidae. Analysis of diadectid interrelationships of all known taxa for which adequate specimens and information are known—the first of its kind conducted—positions Ambedus pusillus as the sister-taxon to all other forms, with Diadectes sanmiguelensis, Orobates pabsti, Desmatodon hesperis, Diadectes absitus, and (Diadectes sideropelicus + Diadectes tenuitectes + Diasparactus zenos) representing progressively more derived taxa in a series of nested clades. In light of these results, it is recommended herein that the species Diadectes sanmiguelensis be referred to the new genus -

Early Tetrapod Relationships Revisited
Biol. Rev. (2003), 78, pp. 251–345. f Cambridge Philosophical Society 251 DOI: 10.1017/S1464793102006103 Printed in the United Kingdom Early tetrapod relationships revisited MARCELLO RUTA1*, MICHAEL I. COATES1 and DONALD L. J. QUICKE2 1 The Department of Organismal Biology and Anatomy, The University of Chicago, 1027 East 57th Street, Chicago, IL 60637-1508, USA ([email protected]; [email protected]) 2 Department of Biology, Imperial College at Silwood Park, Ascot, Berkshire SL57PY, UK and Department of Entomology, The Natural History Museum, Cromwell Road, London SW75BD, UK ([email protected]) (Received 29 November 2001; revised 28 August 2002; accepted 2 September 2002) ABSTRACT In an attempt to investigate differences between the most widely discussed hypotheses of early tetrapod relation- ships, we assembled a new data matrix including 90 taxa coded for 319 cranial and postcranial characters. We have incorporated, where possible, original observations of numerous taxa spread throughout the major tetrapod clades. A stem-based (total-group) definition of Tetrapoda is preferred over apomorphy- and node-based (crown-group) definitions. This definition is operational, since it is based on a formal character analysis. A PAUP* search using a recently implemented version of the parsimony ratchet method yields 64 shortest trees. Differ- ences between these trees concern: (1) the internal relationships of aı¨stopods, the three selected species of which form a trichotomy; (2) the internal relationships of embolomeres, with Archeria -

C.-Shoulder Joint
59.14.98,1:81 Article X.-THE LOCOMOTOR APPARATUS OF CERTAIN PRIMITIVE AND MAMMAL-LIKE REPTILES' BY ALFRED S. ROMER PLATES XXVII TO XLVI TABLE OF CONTENTS PAGE INTRODIUCTION ......................................................... 519 FORE LIMB, MIUSCULATURE ............................................. 524 1.-Trapezius (and sterno-cleido-mastoideus) ........................ 524 2.-Axial muscles acting on the girdle ............................... 525 a.-Dorsal: levator scapul& (and atlanto-scapularis, atlanto- acromialis or omo-trachelius and rhomboideuA), serratus anterior .............................................. 525 b.-Ventral: sterno- (episterno) hyoideus and omo-hyoideus, sterno-costo-coracoidei (subclavius) ................... 526 3.-Latissimus dorsi and teres major ................................ 527 4.-Pectoralis ..................................................... 528 5.-Deltoid ....................................................... 529 6.-Subcoraco-scapularis and scap'ilo-humeralis posterior (subscapularis) 530 7.-Scapulo-humeralis anterior (teres minor), supracoracoideus (supra- and infraspinatus), and coraco-brachialis ..................... 532 8.-Biceps and brachialis .......................................... 536 9.-Triceps ............................... A 537 10.-Brachio- (humero-) radialis (supinator longus) and supinator ...... 538 11.-Forearm: extensors............................................. 539 12.-Forearm: flexors (includingpronators) ............................ 540 13.-Groupingof forelimb muscles -
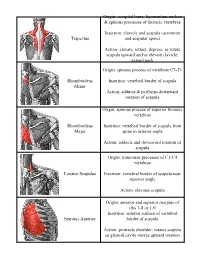
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Systematic Morphology of Fishes in the Early 21St Century
Copeia 103, No. 4, 2015, 858–873 When Tradition Meets Technology: Systematic Morphology of Fishes in the Early 21st Century Eric J. Hilton1, Nalani K. Schnell2, and Peter Konstantinidis1 Many of the primary groups of fishes currently recognized have been established through an iterative process of anatomical study and comparison of fishes that has spanned a time period approaching 500 years. In this paper we give a brief history of the systematic morphology of fishes, focusing on some of the individuals and their works from which we derive our own inspiration. We further discuss what is possible at this point in history in the anatomical study of fishes and speculate on the future of morphology used in the systematics of fishes. Beyond the collection of facts about the anatomy of fishes, morphology remains extremely relevant in the age of molecular data for at least three broad reasons: 1) new techniques for the preparation of specimens allow new data sources to be broadly compared; 2) past morphological analyses, as well as new ideas about interrelationships of fishes (based on both morphological and molecular data) provide rich sources of hypotheses to test with new morphological investigations; and 3) the use of morphological data is not limited to understanding phylogeny and evolution of fishes, but rather is of broad utility to understanding the general biology (including phenotypic adaptation, evolution, ecology, and conservation biology) of fishes. Although in some ways morphology struggles to compete with the lure of molecular data for systematic research, we see the anatomical study of fishes entering into a new and exciting phase of its history because of recent technological and methodological innovations. -

Comparative Anatomy and Functional Morphology University of California at Berkeley Department of Integrative Biology (5 Units)
Comparative Anatomy and Functional Morphology University of California at Berkeley Department of Integrative Biology (5 Units) Course Description: This course is an in-depth look at the biology of form and function. We will examine vertebrate anatomy to understand how structures develop, how they have evolved, and how they interact with one another to allow animals to live in a variety of environments. We will study the integration of the skeletal, muscular, nervous, vascular, respiratory, digestive, endocrine, and urogenital systems to explore the historical and present diversity of vertebrate animals. Format: This course will include lecture, discussion, and laboratory sessions. Lectures will focus primarily on broad concepts and ideas. Discussion sections will emphasize how to both read and analyze primary scientific literature. Laboratory sessions will allow students to physically explore the morphologies of a diversity of taxa and will include museum specimens as well as dissections of whole animals. Lecture: Monday, Tuesday, Wednesday, and Thursday, 9:30-11:00a.m. Discussion: Monday and Thursday, 11:00a.m. or 12:00p.m. Laboratory: Monday, Tuesday, Wednesday, and Thursday, 2:00-5:00p.m. All students must take lectures, discussion sections, and laboratory sections concurrently. Attendance of all sections is required. Goals: 1. A comparative approach will allow students to gain experience in identifying similarities and differences among taxa and further their understanding of how both evolutionary and environmental contexts influence the morphology and function. 2. Students will improve their ability to ask questions and answer them using scientific methodology. 3. Students will gain factual knowledge of terms and concepts regarding vertebrate anatomy and functional morphology.