Deficits in Olfactory Sensitivity in a Mouse Model of Parkinson's
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basal Forebrain Volume Reliably Predicts the Cortical Spread of Alzheimer’S Degeneration
bioRxiv preprint doi: https://doi.org/10.1101/676544; this version posted June 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Title: Basal forebrain volume reliably predicts the cortical spread of Alzheimer’s degeneration Short running title: Predictive spread of Alzheimer’s degeneration Authors: Sara Fernández-Cabello1,2, Martin Kronbichler1,2,3, Koene R. A. Van Dijk4, James A. Goodman4, R. Nathan Spreng5,6,7, Taylor W. Schmitz8,9 for the Alzheimer’s Disease Neuroimaging Initiative* Affiliations: 1 Department of Psychology, University of Salzburg, Salzburg, Austria 2 Centre for Cognitive Neuroscience, University of Salzburg, Salzburg, Austria 3 Neuroscience Institute, Christian-Doppler Medical Centre, Paracelsus Medical University, Salzburg, Austria. 4 Clinical and Translational Imaging, Early Clinical Development, Pfizer Inc, Cambridge, MA, United States 5 Laboratory of Brain and Cognition, Montreal Neurological Institute, Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada 6 Departments of Psychiatry and Psychology, McGill University, Montreal, QC, Canada 7 Douglas Mental Health University Institute, Verdun, QC, Canada 8 Brain and Mind Institute, Western University, London, ON, Canada 9 Department of Physiology and Pharmacology, Western University, London, ON, Canada * Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. -

The Prion-Like Spreading of Alpha-Synuclein in Parkinson’S Disease: Update on Models and Hypotheses
International Journal of Molecular Sciences Review The Prion-Like Spreading of Alpha-Synuclein in Parkinson’s Disease: Update on Models and Hypotheses Asad Jan 1,* ,Nádia Pereira Gonçalves 1,2 , Christian Bjerggaard Vaegter 1,2 , Poul Henning Jensen 1 and Nelson Ferreira 1,* 1 Danish Research Institute of Translational Neuroscience (DANDRITE), Nordic EMBL Partnership for Molecular Medicine, Department of Biomedicine, Aarhus University, 8000 Aarhus, Denmark; [email protected] (N.P.G.); [email protected] (C.B.V.); [email protected] (P.H.J.) 2 International Diabetic Neuropathy Consortium (IDNC), Aarhus University Hospital, 8200 Aarhus, Denmark * Correspondence: [email protected] (A.J.); [email protected] (N.F.) Abstract: The pathological aggregation of the presynaptic protein α-synuclein (α-syn) and propa- gation through synaptically coupled neuroanatomical tracts is increasingly thought to underlie the pathophysiological progression of Parkinson’s disease (PD) and related synucleinopathies. Although the precise molecular mechanisms responsible for the spreading of pathological α-syn accumulation in the CNS are not fully understood, growing evidence suggests that de novo α-syn misfolding and/or neuronal internalization of aggregated α-syn facilitates conformational templating of en- dogenous α-syn monomers in a mechanism reminiscent of prions. A refined understanding of the biochemical and cellular factors mediating the pathological neuron-to-neuron propagation of mis- folded α-syn will potentially elucidate the etiology of PD and unravel novel targets for therapeutic Citation: Jan, A.; Gonçalves, N.P.; intervention. Here, we discuss recent developments on the hypothesis regarding trans-synaptic Vaegter, C.B.; Jensen, P.H.; Ferreira, N. -

(Brainnet Europe Protocol) to the MRC Cognitive Function and Ageing Brain Study Stephen B
Wharton et al. Acta Neuropathologica Communications (2016) 4:11 DOI 10.1186/s40478-016-0275-x RESEARCH Open Access Epidemiological pathology of Tau in the ageing brain: application of staging for neuropil threads (BrainNet Europe protocol) to the MRC cognitive function and ageing brain study Stephen B. Wharton1, Thais Minett2,3, David Drew1, Gillian Forster1, Fiona Matthews4, Carol Brayne3, Paul G. Ince1* and on behalf of the MRC Cognitive Function and Ageing Neuropathology Study Group Abstract Introduction: Deposition of abnormally phosphorylated tau (phospho-tau) occurs in Alzheimer’sdisease but also with brain ageing. The Braak staging scheme focused on neurofibrillary tangles, butabundant p-tau is also present in neuropil threads, and a recent scheme has been proposed by theBrainNet Europe consortium for staging tau pathology based on neuropil threads. We determined therelationship of threads to tangles, and the value of staging for threads in an unselected population-representative ageing brain cohort. We also determined the prevalence of astroglial tau pathologies, and their relationship to neuronal tau. Phospho-tau pathology was determined by immunohistochemistry (AT8 antibody) in the MRC-CFAS neuropathology cohort. Neuropil threads were staged using the BrainNet Europe protocol for tau pathology, and compared with Braak tangle stages. Astroglial tau pathology was assessed in neo-cortical, mesial temporal and subcortical areas. Results: Cases conformed well to the hierarchical neuropil threads staging of the BrainNet Europe protocol and correlated strongly with Braak staging (r=0.84, p < 0.001). Based on the areas under the receiver operator curves (AUC), incorporating either threads or tangle staging significantly improved dementia case identification to a similar degree over age alone (Braak stage X2(1)=10.1, p=0.002; BNE stage X2(1)=9.7, p=0.002). -
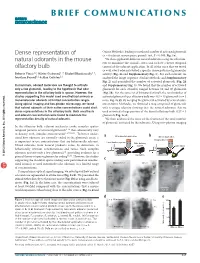
Dense Representation of Natural Odorants in the Mouse Olfactory Bulb
BRIEF COMMUNICATIONS Dense representation of Online Methods), leading to a reduced number of activated glomeruli (n = 6 odorant-mouse pairs, paired t test, P < 0.005; Fig. 1e). natural odorants in the mouse We then applied 40 different natural odorants using the olfactom- eter to minimize the animal’s stress and to have a better temporal olfactory bulb control of the odorant application. In all of the mice that we tested (n = 8), every odorant evoked a specific dense pattern of glomerular Roberto Vincis1,2, Olivier Gschwend1–3, Khaleel Bhaukaurally1–3, activity (Fig. 2a and Supplementary Fig. 1). For each odorant, we Jonathan Beroud1,2 & Alan Carleton1,2 analyzed the image sequence (Online Methods and Supplementary Fig. 2) and quantified the number of activated glomeruli (Fig. 2b In mammals, odorant molecules are thought to activate and Supplementary Fig. 3). We found that the number of activated only a few glomeruli, leading to the hypothesis that odor glomeruli for each stimulus ranged between 10 and 40 glomeruli representation in the olfactory bulb is sparse. However, the (Fig. 2b). For the same set of 30 natural stimuli, the total number of studies supporting this model used anesthetized animals or activated glomeruli per olfactory bulb was 443 ± 15 glomeruli (n = 5 monomolecular odorants at limited concentration ranges. mice; Fig. 2c,d). By merging the glomeruli activated by several odor- Using optical imaging and two-photon microscopy, we found ants (Online Methods), we obtained a map composed of glomeruli that natural odorants at their native concentrations could elicit with a unique identity showing that the natural odorants that we dense representations in the olfactory bulb. -

Alpha-Synuclein: Mechanisms of Release and Pathology Progression in Synucleinopathies
cells Review Alpha-Synuclein: Mechanisms of Release and Pathology Progression in Synucleinopathies Inês C. Brás 1 and Tiago F. Outeiro 1,2,3,4,* 1 Center for Biostructural Imaging of Neurodegeneration, Department of Experimental Neurodegeneration, University Medical Center Göttingen, 37075 Göttingen, Germany; [email protected] 2 Max Planck Institute for Experimental Medicine, 37075 Göttingen, Germany 3 Faculty of Medical Sciences, Translational and Clinical Research Institute, Newcastle University, Framlington Place, Newcastle Upon Tyne NE2 4HH, UK 4 Scientific Employee with a Honorary Contract at Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), 37075 Göttingen, Germany * Correspondence: [email protected]; Tel.: +49-(0)-551-391-3544; Fax: +49-(0)-551-392-2693 Abstract: The accumulation of misfolded alpha-synuclein (aSyn) throughout the brain, as Lewy pathology, is a phenomenon central to Parkinson’s disease (PD) pathogenesis. The stereotypical distribution and evolution of the pathology during disease is often attributed to the cell-to-cell transmission of aSyn between interconnected brain regions. The spreading of conformationally distinct aSyn protein assemblies, commonly referred as strains, is thought to result in a variety of clin- ically and pathologically heterogenous diseases known as synucleinopathies. Although tremendous progress has been made in the field, the mechanisms involved in the transfer of these assemblies between interconnected neural networks and their role in driving PD progression are still unclear. Here, we present an update of the relevant discoveries supporting or challenging the prion-like spreading hypothesis. We also discuss the importance of aSyn strains in pathology progression and the various putative molecular mechanisms involved in cell-to-cell protein release. Understanding Citation: Brás, I.C.; Outeiro, T.F. -

Taste and Smell Disorders in Clinical Neurology
TASTE AND SMELL DISORDERS IN CLINICAL NEUROLOGY OUTLINE A. Anatomy and Physiology of the Taste and Smell System B. Quantifying Chemosensory Disturbances C. Common Neurological and Medical Disorders causing Primary Smell Impairment with Secondary Loss of Food Flavors a. Post Traumatic Anosmia b. Medications (prescribed & over the counter) c. Alcohol Abuse d. Neurodegenerative Disorders e. Multiple Sclerosis f. Migraine g. Chronic Medical Disorders (liver and kidney disease, thyroid deficiency, Diabetes). D. Common Neurological and Medical Disorders Causing a Primary Taste disorder with usually Normal Olfactory Function. a. Medications (prescribed and over the counter), b. Toxins (smoking and Radiation Treatments) c. Chronic medical Disorders ( Liver and Kidney Disease, Hypothyroidism, GERD, Diabetes,) d. Neurological Disorders( Bell’s Palsy, Stroke, MS,) e. Intubation during an emergency or for general anesthesia. E. Abnormal Smells and Tastes (Dysosmia and Dysgeusia): Diagnosis and Treatment F. Morbidity of Smell and Taste Impairment. G. Treatment of Smell and Taste Impairment (Education, Counseling ,Changes in Food Preparation) H. Role of Smell Testing in the Diagnosis of Neurodegenerative Disorders 1 BACKGROUND Disorders of taste and smell play a very important role in many neurological conditions such as; head trauma, facial and trigeminal nerve impairment, and many neurodegenerative disorders such as Alzheimer’s, Parkinson Disorders, Lewy Body Disease and Frontal Temporal Dementia. Impaired smell and taste impairs quality of life such as loss of food enjoyment, weight loss or weight gain, decreased appetite and safety concerns such as inability to smell smoke, gas, spoiled food and one’s body odor. Dysosmia and Dysgeusia are very unpleasant disorders that often accompany smell and taste impairments. -

Review Article Olfactory Reference Syndrome
British Journal of Pharmaceutical and Medical Research Vol.04, Issue 01, Pg.1617-1625, January-February 2019 Available Online at http://www.bjpmr.org BRITISH JOURNAL OF PHARMACEUTICAL AND MEDICAL RESEARCH Review ISSN:2456-9836 ICV: 60.37 Article Murat Eren Özen, Murat Aydin Olfactory Reference Syndrome: A Separate Disorder Or Part Of A Spectrum 1Psychiatrist, Department of Psychiatry, Private Adana Hospital, Adana Büyük şehir Belediyesi kar şısı, No:23, Seyhan-Adana- Türkiye. 2Private Dental Clinics, Gazipa şa bulv. Emre apt n:6 (kitapsan kar şısı) k:2 d:5 Adana- Türkiye. http://drmurataydin.com ARTICLE INFO ABSTRACT This article provides a narrative review of the literature on olfactory reference syndrome Article History: Received on (ORS) to address issues focusing on its clinical features. Similarities and/or differences with other psychiatric disorders such as obsessive-compulsive spectrum disorders, social anxiety 10 th Jan, 2019 Peer Reviewed disorder (including a cultural syndrome; taijin kyofusho), somatoform disorders and hypochondriasis, delusional disorder are discussed. ORS is related to a symptom of taijin on 24 th Jan, 2019 Revised kyofusho (e.g. jikoshu-kyofu variant of taijin kyofusho) Although recognition of this syndromes more than a century provide consistent descriptions of its clinical features, the on 17 th Feb, 2019 Published limited data on this topic make it difficult to form a specific diagnostic criteria. The core on 24 th Feb, symptom of the patients with ORS is preoccupation with the belief that one emits a foul or 2019 offensive body odor, which is not perceived by others. Studies on ORS reveal some limitations. -

The Consequences of Pretangle Tau in the Locus Coeruleus Termpanit Chalermpalanupap, Emory University David Weinshenker, Emory University Jacki M
Down but Not Out: The Consequences of Pretangle Tau in the Locus Coeruleus Termpanit Chalermpalanupap, Emory University David Weinshenker, Emory University Jacki M. Rorabaugh, Emory University Journal Title: Neural Plasticity Volume: Volume 2017 Publisher: Hindawi Publishing Corporation | 2017-09-05, Pages 7829507-7829507 Type of Work: Article | Final Publisher PDF Publisher DOI: 10.1155/2017/7829507 Permanent URL: https://pid.emory.edu/ark:/25593/s64c4 Final published version: http://dx.doi.org/10.1155/2017/7829507 Copyright information: © 2017 Termpanit Chalermpalanupap et al. This is an Open Access work distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/). Accessed September 29, 2021 12:12 PM EDT Hindawi Neural Plasticity Volume 2017, Article ID 7829507, 9 pages https://doi.org/10.1155/2017/7829507 Review Article Down but Not Out: The Consequences of Pretangle Tau in the Locus Coeruleus Termpanit Chalermpalanupap, David Weinshenker, and Jacki M. Rorabaugh Department of Human Genetics, Emory University School of Medicine, Atlanta, GA 30322, USA Correspondence should be addressed to Jacki M. Rorabaugh; [email protected] Received 10 March 2017; Revised 20 June 2017; Accepted 20 July 2017; Published 5 September 2017 Academic Editor: Niels Hansen Copyright © 2017 Termpanit Chalermpalanupap et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Degeneration of locus coeruleus (LC) is an underappreciated hallmark of Alzheimer’s disease (AD). The LC is the main source of norepinephrine (NE) in the forebrain, and its degeneration is highly correlated with cognitive impairment and amyloid-beta (Aβ) and tangle pathology. -

The Unfolded Protein Response Is Activated in the Olfactory System in Alzheimer’S Disease Helen C
Murray et al. Acta Neuropathologica Communications (2020) 8:109 https://doi.org/10.1186/s40478-020-00986-7 RESEARCH Open Access The unfolded protein response is activated in the olfactory system in Alzheimer’s disease Helen C. Murray1,2* , Birger Victor Dieriks1, Molly E. V. Swanson1, Praju Vikas Anekal1, Clinton Turner3, Richard L. M. Faull1, Leonardo Belluscio4, Alan Koretsky2 and Maurice A. Curtis1* Abstract Olfactory dysfunction is an early and prevalent symptom of Alzheimer’s disease (AD) and the olfactory bulb is a nexus of beta-amyloid plaque and tau neurofibrillary tangle (NFT) pathology during early AD progression. To mitigate the accumulation of misfolded proteins, an endoplasmic reticulum stress response called the unfolded protein response (UPR) occurs in the AD hippocampus. However, chronic UPR activation can lead to apoptosis and the upregulation of beta-amyloid and tau production. Therefore, UPR activation in the olfactory system could be one of the first changes in AD. In this study, we investigated whether two proteins that signal UPR activation are expressed in the olfactory system of AD cases with low or high amounts of aggregate pathology. We used immunohistochemistry to label two markers of UPR activation (p-PERK and p-eIF2α) concomitantly with neuronal markers (NeuN and PGP9.5) and pathology markers (beta-amyloid and tau) in the olfactory bulb, piriform cortex, entorhinal cortex and the CA1 region of the hippocampus in AD and normal cases. We show that UPR activation, as indicated by p-PERK and p-eIF2α expression, is significantly increased throughout the olfactory system in AD cases with low (Braak stage III-IV) and high-level (Braak stage V-VI) pathology. -

Single Olfactory Receptors Set Odor Detection Thresholds
City University of New York (CUNY) CUNY Academic Works Publications and Research Hunter College 2018 Single olfactory receptors set odor detection thresholds Adam Dewan Northwestern University Annika Cichy Northwestern University Jingji Zhang Northwestern University Kayla Miguel Northwestern University Paul Feinstein CUNY Hunter College See next page for additional authors How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/hc_pubs/550 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Authors Adam Dewan, Annika Cichy, Jingji Zhang, Kayla Miguel, Paul Feinstein, Dmitry Rinberg, and Thomas Bozza This article is available at CUNY Academic Works: https://academicworks.cuny.edu/hc_pubs/550 ARTICLE DOI: 10.1038/s41467-018-05129-0 OPEN Single olfactory receptors set odor detection thresholds Adam Dewan1, Annika Cichy1, Jingji Zhang1, Kayla Miguel1, Paul Feinstein2, Dmitry Rinberg3 & Thomas Bozza 1 In many species, survival depends on olfaction, yet the mechanisms that underlie olfactory sensitivity are not well understood. Here we examine how a conserved subset of olfactory receptors, the trace amine-associated receptors (TAARs), determine odor detection fi 1234567890():,; thresholds of mice to amines. We nd that deleting all TAARs, or even single TAARs, results in significant odor detection deficits. This finding is not limited to TAARs, as the deletion of a canonical odorant receptor reduced behavioral sensitivity to its preferred ligand. Remarkably, behavioral threshold is set solely by the most sensitive receptor, with no contribution from other highly sensitive receptors. -

Respiration, Neural Oscillations, and the Free Energy Principle
fnins-15-647579 June 23, 2021 Time: 17:40 # 1 REVIEW published: 29 June 2021 doi: 10.3389/fnins.2021.647579 Keeping the Breath in Mind: Respiration, Neural Oscillations, and the Free Energy Principle Asena Boyadzhieva1*† and Ezgi Kayhan2,3† 1 Department of Philosophy, University of Vienna, Vienna, Austria, 2 Department of Developmental Psychology, University of Potsdam, Potsdam, Germany, 3 Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany Scientific interest in the brain and body interactions has been surging in recent years. One fundamental yet underexplored aspect of brain and body interactions is the link between the respiratory and the nervous systems. In this article, we give an overview of the emerging literature on how respiration modulates neural, cognitive and emotional processes. Moreover, we present a perspective linking respiration to the free-energy principle. We frame volitional modulation of the breath as an active inference mechanism Edited by: in which sensory evidence is recontextualized to alter interoceptive models. We further Yoko Nagai, propose that respiration-entrained gamma oscillations may reflect the propagation of Brighton and Sussex Medical School, United Kingdom prediction errors from the sensory level up to cortical regions in order to alter higher level Reviewed by: predictions. Accordingly, controlled breathing emerges as an easily accessible tool for Rishi R. Dhingra, emotional, cognitive, and physiological regulation. University of Melbourne, Australia Georgios D. Mitsis, Keywords: interoception, respiration-entrained neural oscillations, controlled breathing, free-energy principle, McGill University, Canada self-regulation *Correspondence: Asena Boyadzhieva [email protected] INTRODUCTION † These authors have contributed “The “I think” which Kant said must be able to accompany all my objects, is the “I breathe” which equally to this work actually does accompany them. -

Staging of Alzheimer Disease-Associated Neurowbrillary Pathology Using Parayn Sections and Immunocytochemistry
Acta Neuropathol (2006) 112:389–404 DOI 10.1007/s00401-006-0127-z METHODS REPORT Staging of Alzheimer disease-associated neuroWbrillary pathology using paraYn sections and immunocytochemistry Heiko Braak · Irina AlafuzoV · Thomas Arzberger · Hans Kretzschmar · Kelly Del Tredici Received: 8 June 2006 / Revised: 21 July 2006 / Accepted: 21 July 2006 / Published online: 12 August 2006 © Springer-Verlag 2006 Abstract Assessment of Alzheimer’s disease (AD)- revised here by adapting tissue selection and process- related neuroWbrillary pathology requires a procedure ing to the needs of paraYn-embedded sections (5–15 m) that permits a suYcient diVerentiation between initial, and by introducing a robust immunoreaction (AT8) for intermediate, and late stages. The gradual deposition hyperphosphorylated tau protein that can be processed of a hyperphosphorylated tau protein within select on an automated basis. It is anticipated that this neuronal types in speciWc nuclei or areas is central to revised methodological protocol will enable a more the disease process. The staging of AD-related neuroW- uniform application of the staging procedure. brillary pathology originally described in 1991 was per- formed on unconventionally thick sections (100 m) Keywords Alzheimer’s disease · NeuroWbrillary using a modern silver technique and reXected the pro- changes · Immunocytochemistry · gress of the disease process based chieXy on the topo- Hyperphosphorylated tau protein · Neuropathologic graphic expansion of the lesions. To better meet the staging · Pretangles demands of routine laboratories this procedure is Introduction This study was made possible by funding from the German Research Council (Deutsche Forschungsgemeinschaft) and BrainNet Europe II (European Commission LSHM-CT-2004- The development of intraneuronal lesions at selec- 503039).