Case Report Gestational Diabetes Insipidus Associated with HELLP Syndrome: Acasereport
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Porphyromonas Gingivalis Within Placental Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome
RESEARCH ARTICLE Porphyromonas gingivalis within Placental Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome Sizzle F. Vanterpool1,2, Jasper V. Been1,3,4, Michiel L. Houben5, Peter G. J. Nikkels6, Ronald R. De Krijger7, Luc J. I. Zimmermann1,8, Boris W. Kramer1,2,8, Ann Progulske-Fox9, Leticia Reyes9,10* 1 Department of Pediatrics, Maastricht University Medical Center, Maastricht, the Netherlands, 2 School for Mental Health and Neurosciences (MHeNS), Maastricht University, Maastricht, the Netherlands, 3 School for Public Health and Primary Care (CAPHRI), Maastricht University, Maastricht, the Netherlands, 4 Division of Neonatology, Erasmus University Medical Center–Sophia Children’s Hospital, Rotterdam, the Netherlands, 5 Department of Pediatrics, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, the Netherlands, 6 Department of Pathology, University Medical Center Utrecht, Utrecht, the Netherlands, 7 Department of Pathology, Erasmus University Medical Center, Rotterdam, the Netherlands, 8 School for Oncology and Developmental Biology (GROW), Maastricht University, Maastricht, the Netherlands, OPEN ACCESS 9 Department of Oral Biology, Center for Molecular Microbiology, University of Florida, Gainesville, Florida, Citation: Vanterpool SF, Been JV, Houben ML, United States of America, 10 Department of Pathobiological Sciences, University of Wisconsin-Madison, Nikkels PGJ, De Krijger RR, Zimmermann LJI, et al. Madison, Wisconsin, United States of America (2016) Porphyromonas gingivalis within Placental * [email protected] Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome. PLoS ONE 11(1): e0146157. doi:10.1371/journal. pone.0146157 Abstract Editor: Motohiro Komaki, Tokyo Medical and Dental University, JAPAN Intrauterine presence of Porphyromonas gingivalis (Pg), a common oral pathobiont, is impli- cated in preterm birth. -

Gestational Diabetes Insipidus, HELLP Syndrome and Eclampsia in a Twin Pregnancy: a Case Report
Journal of Perinatology (2010) 30, 144–145 r 2010 Nature Publishing Group All rights reserved. 0743-8346/10 $32 www.nature.com/jp PERINATAL/NEONATAL CASE PRESENTATION Gestational diabetes insipidus, HELLP syndrome and eclampsia in a twin pregnancy: a case report JL Woelk, RA Dombroski and PR Brezina Department of Obstetrics and Gynecology, East Carolina University, Greenville, NC, USA alanine aminotransferase, 65 U lÀ1; and lactate dehydrogenase, We report a case of eclampsia in a twin pregnancy complicated by HELLP 390 U lÀ1. A 24-h urine collection was begun to quantify proteinuria. syndrome and diabetes insipidus. This confluence of disease processes During the first night of hospitalization, the patient developed suggests that a modification of common magnesium sulfate treatment marked polyuria and polydipsia. Urine output increased to 1500 cc hÀ1 protocols may be appropriate in a certain subset of patients. by the early morning totaling 12 l for the entire night. Repeat Journal of Perinatology (2010) 30, 144–145; doi:10.1038/jp.2009.115 electrolytes were unchanged. The endocrinology service was then Keywords: diabetes insipidus ; eclampsia ; HELLP syndrome ; twin consulted for the management of presumptive DI. Therapy with the pregnancy ; magnesium sulfate administration of dDAVP (1-deamino-8-D-arginine vasopressin) orally twice daily was initiated. Serum osmolality was increased at 296 mOsm kgÀ1, and urine osmolality was decreased to 71 mOsm kgÀ1 Introduction with a specific gravity of 1.000. The 24-h urine results showed a total The association between diabetes insipidus (DI) and liver protein of 780 mg. The patient denied the previously reported dysfunction in a preeclamptic patient has been established in headaches, blurry vision and right upper quadrant pain, and her blood multiple case reports.1 This case is an important example that pressures remained 140 to 155 mmHg (systolic) and 80 to 95 mmHg illustrates how the pathophysiology of DI and the pharmacokinetics (diastolic), consistent with a diagnosis of mild preeclampsia. -

Incidence of Eclampsia with HELLP Syndrome and Associated Mortality in Latin America
International Journal of Gynecology and Obstetrics 129 (2015) 219–222 Contents lists available at ScienceDirect International Journal of Gynecology and Obstetrics journal homepage: www.elsevier.com/locate/ijgo CLINICAL ARTICLE Incidence of eclampsia with HELLP syndrome and associated mortality in Latin America Paulino Vigil-De Gracia a,⁎, José Rojas-Suarez b, Edwin Ramos c, Osvaldo Reyes d, Jorge Collantes e, Arelys Quintero f,ErasmoHuertasg, Andrés Calle h, Eduardo Turcios i,VicenteY.Chonj a Critical Care Unit, Department of Obstetrics and Gynecology, Complejo Hospitalario de la Caja de Seguro Social, Panama City, Panama b Critical Care Unit, Clínica de Maternidad Rafael Calvo, Cartagena, Colombia c Department of Gynecology and Obstetrics, Hospital Universitario Dr Luis Razetti, Barcelona, Venezuela d Unit of Research, Department of Gynecology and Obstetrics, Hospital Santo Tomás, Panama City, Panama e Department of Gynecology and Obstetrics, Hospital Regional de Cojamarca, Cajamarca, Peru f Department of Gynecology and Obstetrics, Hospital José Domingo de Obaldía, David, Panama g Unit of Perinatology, Department of Gynecology and Obstetrics, Instituto Nacional Materno Perinatal, Lima, Peru h Department of Gynecology and Obstetrics, Hospital Carlos Andrade Marín, Quito, Ecuador i Unit of Research, Department of Gynecology and Obstetrics, Hospital Primero de Mayo de Seguridad Social, San Salvador, El Salvador j Department of Gynecology and Obstetrics, Hospital Teodoro Maldonado Carbo, Guayaquil, Ecuador article info abstract Article history: Objective: To describe the maternal outcome among women with eclampsia with and without HELLP syndrome Received 7 July 2014 (hemolysis, elevated liver enzymes, and low platelet count). Methods: A cross-sectional study of women with Received in revised form 14 November 2014 eclampsia was undertaken in 14 maternity units in Latin America between January 1 and December 31, 2012. -
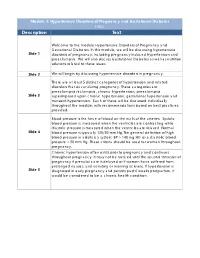
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text Welcome to the module Hypertensive Disorders of Pregnancy and Gestational Diabetes. In this module, we will be discussing hypertensive Slide 1 disorders of pregnancy, including pregnancy induced hypertension and preeclampsia. We will also discuss Gestational Diabetes as well as nutrition solutions related to these issues Slide 2 We will begin by discussing hypertensive disorders in pregnancy. There are at least 5 distinct categories of hypertension and related disorders that occur during pregnancy. These categories are: preeclampsia/eclampsia, chronic hypertension, preeclampsia Slide 3 superimposed upon chronic hypertension, gestational hypertension and transient hypertension. Each of these will be discussed individually throughout the module, with recommendations based on best practices provided. Blood pressure is the force of blood on the walls of the arteries. Systolic blood pressure is measured when the ventricles are contracting while diastolic pressure is measured when the ventricles are relaxed. Normal Slide 4 blood pressure is typically 120/80 mm Hg.The general definition of high blood pressure in adults is a systolic BP > 140 mg HG or a diastolic blood pressure > 90 mm Hg. These criteria should be used for women throughout pregnancy. Chronic hypertension often exists prior to pregnancy and continues throughout pregnancy. It may not be noticed until the second trimester of pregnancy if prenatal care is delayed or if women have suffered from prolonged nausea and vomiting or morning sickness. If hypertension is Slide 5 diagnosed in early pregnancy and persists past 6 weeks postpartum, it would be considered to be a chronic health condition. -

Role of Maternal Age and Pregnancy History in Risk of Miscarriage
RESEARCH Role of maternal age and pregnancy history in risk of BMJ: first published as 10.1136/bmj.l869 on 20 March 2019. Downloaded from miscarriage: prospective register based study Maria C Magnus,1,2,3 Allen J Wilcox,1,4 Nils-Halvdan Morken,1,5,6 Clarice R Weinberg,7 Siri E Håberg1 1Centre for Fertility and Health, ABSTRACT Miscarriage and other pregnancy complications might Norwegian Institute of Public OBJECTIVES share underlying causes, which could be biological Health, PO Box 222 Skøyen, To estimate the burden of miscarriage in the conditions or unmeasured common risk factors. N-0213 Oslo, Norway Norwegian population and to evaluate the 2MRC Integrative Epidemiology associations with maternal age and pregnancy history. Unit at the University of Bristol, Introduction Bristol, UK DESIGN 3 Miscarriage is a common outcome of pregnancy, Department of Population Prospective register based study. Health Sciences, Bristol Medical with most studies reporting 12% to 15% loss among School, Bristol, UK SETTING recognised pregnancies by 20 weeks of gestation.1-4 4Epidemiology Branch, National Medical Birth Register of Norway, the Norwegian Quantifying the full burden of miscarriage is Institute of Environmental Patient Register, and the induced abortion register. challenging because rates of pregnancy loss are Health Sciences, Durham, NC, USA PARTICIPANTS high around the time that pregnancies are clinically 5Department of Clinical Science, All Norwegian women that were pregnant between recognised. As a result, the total rate of recognised University of Bergen, Bergen, 2009-13. loss is sensitive to how early women recognise their Norway pregnancies. There are also differences across countries 6 MAIN OUTCOME MEASURE Department of Obstetrics and studies in distinguishing between miscarriage and and Gynecology, Haukeland Risk of miscarriage according to the woman’s age and University Hospital, Bergen, pregnancy history estimated by logistic regression. -

Ophthalmic Associations in Pregnancy
CLINICAL Ophthalmic associations in pregnancy Queena Qin, Celia Chen, Sudha Cugati PREGNANCY RESULTS in various physiological variation in pregnancy.2 It normally changes in the female body, including in fades slowly after pregnancy and does the eyes. A typical pregnancy results in not need active intervention. Background A range of ocular pathology exists cardiovascular, pulmonary, metabolic, • Cornea – corneal thickness, curvature during pregnancy. Some pre-existing eye hormonal and immunological changes. and sensitivity may be altered during conditions, such as diabetic retinopathy, Hormonal changes occur, with a rise of pregnancy. Corneal thickness and can be exacerbated during pregnancy. oestrogen and progesterone levels to curvature can increase in pregnancy, Other conditions manifest for the first suppress the menstrual cycle.1 especially in the second and third time during pregnancy as a result of The eye, an end organ, undergoes trimesters, and return to normal in complications such as pre-eclampsia changes during pregnancy. Some of the postpartum period.3 Patients who and eclampsia. Early recognition and understanding of the management of these changes exacerbate pre-existing wear contact lenses may experience ophthalmic conditions is crucial. eye conditions, while other conditions intolerance to the use of contact lenses. manifest for the first time during Pregnant women should be advised Objective pregnancy. Early recognition and to delay obtaining a new prescription The aim of this article is to discuss the understanding of management of for glasses or undergoing a contact physiological and pathological changes in the eyes of pregnant women. ophthalmic conditions during pregnancy lens fitting until after delivery. Laser Pathological changes are sub-divided is crucial for the primary care physician. -

Gestational Diabetes Insipidus (GDI) Associated with Pre-Eclampsia
MOJ Women’s Health Case Report Open Access Gestational diabetes insipidus (GDI) associated with pre-eclampsia Abstract Volume 5 Issue 6 - 2017 Gestational diabetes insipidus (GDI) is a rare complication of pregnancy, usually developing in the third trimester and remitting spontaneously 4-6weeks post-partum. Afsoon Razavi, Muhammad Umair, Zehra It is mainly caused by excessive vasopressinase activity, an enzyme expressed by Tekin, Issac Sachmechi placental trophoblasts which metabolizes arginine vasopressin (AVP). The treatment Department of medicine, Icahn School of Medicine at Mount requires desmopressin. A 38year old G3P0A2 women with no significant medical Sinai/NYC Health+ Hospital/Queens, USA history was admitted to obstetrical service on 36th week of gestation due to significant malaise, anorexia, nausea, vomiting, polyuria, nocturia, and polydipsia, worsening Correspondence: Afsoon Razavi, MD, Department of in the 2weeks prior to presentation. Physical examination demonstrated decreased Medicine, Icahn School of Medicine at Mount Sinai/NYC skin turgor, hyperactive tendon reflexes and no pedal edema, and her blood pressure Health+Hospital/Queens, Diabetes center, 4th floor, Suit P-432, Pavilion building, Queens hospital center, 82-68 164th street, was 170/100mmHg, heart rate 67beats/min and weight 60kg (BMI 23.1kg/m2). Her Jamaica, New York, 11432, USA, Tel +8183843165, laboratory results a month prior to admission showed normal basic metabolic panel Email [email protected] and liver function tests. On admission she found to have urine osmolality112mOsmol/ kg (350-1000); serum osmolality 308mOsmol/kg (278-295); Urinalysis revealed Received: July 25, 2017 | Published: August 16, 2017 specific gravity less than 1005 with proteinuria. serum sodium 151mmol/L (135-145); potassium 4.1mmol/L (3.5-5.0); Cl 128mmol/L, urea 2.2 mmol/L (2.5-6.7), creatinine 1.4mg/dL, Bilirubin 1.3mg/dL, AST 1270 U/L, alkaline phosphatase, 717U/L, uric acid 10.1mg/dL and INR 1.1. -

Critical Care Issues in Pregnancy
CriticalCritical CareCare IssuesIssues inin PregnancyPregnancy Miren A. Schinco, MD, FCCS, FCCM Associate Professor of Surgery University of Florida College of Medicine, Jacksonville College of Medicine – Jacksonville Department of Surgery EpidemiologyEpidemiology •Approximately .1% of deliveries result in ICU admission • Generally, 75% - 80 % are during the post- partum period College of Medicine – Jacksonville Department of Surgery TopTop causescauses ofof mortalitymortality inin obstetricobstetric patientspatients admittedadmitted toto thethe ICUICU Etiology N (of 1354) Percentage Hypertension 20 21.5 Pulmonary 20 21.5 Cardiac 11 11.8 Hemorrhage 8 8.6 CNS 8 8.6 Sepsis/Infection 6 6.4 Malignancy 6 6.4 College of Medicine – Jacksonville Department of Surgery CriticalCritical illnessesillnesses inin pregnancypregnancy A. Conditions unique to pregnancy: account for 50-80% admissions to ICU(account for > 50% ICU admissions): • Preeclampsia / Eclampsia • HELLP syndrome • Acute fatty liver of pregnancy • Amniotic fluid embolism • Peri-partum cardiomyopathy • Puerperal sepsis • Thrombotic disease • Obstetric hemorrhage College of Medicine – Jacksonville Department of Surgery CriticalCritical illnessesillnesses inin pregnancypregnancy B. Pre-existing conditions that may worsen during pregnancy (account for 20-50% ICU admissions): • Cardiovascular: valvular disease, Eisenmenger’s syndrome, cyanotic congenital heart disease, coarctation of aorta, PPH • Renal: glomerulonephritis, chronic renal insufficiency • Hematologic: sickle cell disease, -

Prioritization Changes
Oregon Health Plan Prioritized List changes Planned Out-of-hospital Birth The Health Evidence Review Commission approved the following changes to the Prioritized List of Health Services on August 13, 2020, based on the approved coverage guidance, “Planned Out-of- hospital Birth.” The changes will take effect on the Prioritized list of Health Services for the Oregon Health Plan on October 1, 2020. Changes for the Prioritized List of Health Services: GUIDELINE NOTE 153 PLANNED OUT-OF-HOSPITAL BIRTH Lines 1,2 Planned out-of-hospital birth is included on this line for pregnant women who are at low risk for adverse obstetric or birth outcomes. The high-risk conditions outlined below would either preclude coverage of planned out-of-hospital birth, necessitate a consultation, or require transfer of the mother or infant to a hospital setting. When a condition requiring transfer arises during labor, an attempt should be made to transfer the mother and/or her newborn; however, imminent fetal delivery may delay or preclude actual transfer prior to birth. Coverage of prenatal, intrapartum, and postpartum care is recommended with the performance of appropriate risk assessments (at initiation of care and throughout pregnancy and delivery) and the out- of-hospital birth attendant’s adherence to the consultation and transfer criteria as outlined below. When a high-risk condition develops that requires transfer or planned hospital birth, coverage is recommended when appropriate care is provided until the point the high-risk condition is identified. For women who have a high-risk condition requiring consultation, ongoing coverage of planned out-of- hospital birth care is recommended as long as the consulting provider’s recommendations are then appropriately managed by the out-of-hospital birth attendant in a planned out-of-hospital birth setting. -

1 Supplementary Table S2. Summary of the Results of Systematic Literature
Supplementary Table S2. Summary of the results of systematic literature review Question 1. What is the evidence that the following pre-conception (planned versus unintended pregnancy, disease activity and damage, antibody profile, lupus medications, management of flares, access to specialist services, immunization status and update) and post-conception/delivery (parenting issues, lupus medications, management of flares, access to specialist care) factors influence maternal and foetal outcomes, and thus, should be considered in pregnancy risk stratification and counselling in women with SLE or APS? 1. Women with SLE 1.1. Pre-conception factors associated maternal outcomes Factor/predictor Outcome(s) and example(s) of effect size Best study References design1 Active/flaring SLE (before Active/flaring SLE during pregnancy. SLE flares during the pre-gestation period had OR 4 1-21 or at conception) 5.1 for flare during pregnancy; previous (within 6 months before conception) organ involvement predicted same organ involvement during pregnancy (especially haematological, renal, skin, serological activity); SLE flares during the pre-gestation period had OR 5.1 for development of flare during pregnancy; SLEDAI ≥4 at conception had sensitivity 64% and specificity 75% for SLE exacerbation during pregnancy; remission during the 6-month period prior to conception is associated with lower odds for flare during pregnancy Organ damage. Pre-gestational SLE activity (SLAM-R index) correlates with post-partum 5 22 damage accrual Pregnancy-induced hypertension, (pre-)eclampsia/HELLP. Positive correlation with 4 12 14 21 23 pre-conception SLEDAI; active SLE within 4 months before conception was associated with pregnancy-induced hypertension (25% versus 11%) Adverse maternal outcome (composite outcome or death). -

A B C Pregnancy Terms and Definitions
Pregnancy Terms and Definitions Obstetrics & Gynecology A After pains or afterbirth pains: Contractions of the uterus that occur after your baby is born, as the uterus returns to its normal size. This may cause cramping for a few days, especially if this is not your first baby or if you are nursing. Amniocentesis: the removal of a sample of amniotic fluid by means of a needle inserted through the mother’s abdominal wall; used for genetic and biochemical analysis of the baby. Amniotic fluid: the liquid surrounding and protecting the baby within the amniotic sac throughout pregnancy. Amniotic sac: the membrane within the uterus that contains the baby and the amniotic fluid. Analgesic: Medication that relieves or reduces pain. Anesthesia: Loss of feeling. There are three ways of doing this: general, local and epidural. Anesthesiologist: A doctor who specializes in the use of anesthesia. Anesthetist: A registered nurse who has special training in anesthesia. Apgar score rating: A system to evaluate the health of your baby immediately after birth. The score can be zero to 10, based on appearance and color, pulse, reflexes, activity and respiration. B Baby blues: A mild depression many women feel in the first few weeks after birth. Braxton-Hicks contractions: Mild, usually painless contractions that occur during the entire pregnancy, but are only felt from the 5th month on. Breech birth: Baby is born feet or buttocks first. C Cephalopelvic disproprition (CPD): Baby’s head is too large for the mother’s pelvic bones. Cervix: the neck of the uterus; Pap smears are taken from the cervix. -

Standards of Medical Care in Diabetes—2018
Diabetes Care Volume 41, Supplement 1, January 2018 S137 13. Management of Diabetes American Diabetes Association in Pregnancy: Standards of Medical Care in Diabetesd2018 Diabetes Care 2018;41(Suppl. 1):S137–S143 | https://doi.org/10.2337/dc18-S013 13. MANAGEMENT OF DIABETES IN PREGNANCY The American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations, please refer to the Standards of Care Introduction. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC. DIABETES IN PREGNANCY The prevalence of diabetes in pregnancy has been increasing in the U.S. The majority is gestational diabetes mellitus (GDM) with the remainder primarily preexisting type 1 diabetes and type 2 diabetes. The rise in GDM and type 2 diabetes in parallel with obesity both in the U.S. and worldwide is of particular concern. Both type 1 diabetes and type 2 diabetes in pregnancy confer significantly greater maternal and fetal risk than GDM, with some differences according to type of diabetes as outlined below. In general, specific risks of uncontrolled diabetes in pregnancy include spontaneous abortion, fetal anomalies, preeclampsia, fetal demise, macrosomia, neonatal hy- poglycemia, and neonatal hyperbilirubinemia, among others.