Balearic Shearwater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Population Estimates of a Critically Endangered Species, the Balearic Shearwater Puffinus Mauretanicus , Based on Coastal Migration Counts
Bird Conservation International (2016) 26 :87 –99 . © BirdLife International, 2014 doi:10.1017/S095927091400032X New population estimates of a critically endangered species, the Balearic Shearwater Puffinus mauretanicus , based on coastal migration counts GONZALO M. ARROYO , MARÍA MATEOS-RODRÍGUEZ , ANTONIO R. MUÑOZ , ANDRÉS DE LA CRUZ , DAVID CUENCA and ALEJANDRO ONRUBIA Summary The Balearic Shearwater Puffinus mauretanicus is considered one of the most threatened seabirds in the world, with the breeding population thought to be in the range of 2,000–3,200 breeding pairs, from which global population has been inferred as 10,000 to 15,000 birds. To test whether the actual population of Balearic Shearwaters is larger than presently thought, we analysed the data from four land-based census campaigns of Balearic Shearwater post-breeding migration through the Strait of Gibraltar (mid-May to mid-July 2007–2010). The raw results of the counts, covering from 37% to 67% of the daylight time throughout the migratory period, all revealed figures in excess of 12,000 birds, and went up to almost 18,000 in two years. Generalised Additive Models were used to estimate the numbers of birds passing during the time periods in which counts were not undertaken (count gaps), and their associated error. The addition of both counted and esti- mated birds reveals figures of between 23,780 and 26,535 Balearic Shearwaters migrating along the north coast of the Strait of Gibraltar in each of the four years of our study. The effects of several sources of bias suggest a slight potential underestimation in our results. -

Recording the Manx Shearwater
RECORDING THE MANX There Kennedy and Doctor Blair, SHEARWATER Tall Alan and John stout There Min and Joan and Sammy eke Being an account of Dr. Ludwig Koch's And Knocks stood all about. adventures in the Isles of Scilly in the year of our Lord nineteen “ Rest, Ludwig, rest," the doctor said, hundred and fifty one, in the month of But Ludwig he said "NO! " June. This weather fine I dare not waste, To Annet I will go. This very night I'll records make, (If so the birds are there), Of Shearwaters* beneath the sod And also in the air." So straight to Annet's shores they sped And straight their task began As with a will they set ashore Each package and each man Then man—and woman—bent their backs And struggled up the rock To where his apparatus was Set up by Ludwig Koch. And some the heavy gear lugged up And some the line deployed, Until the arduous task was done And microphone employed. Then Ludwig to St. Agnes hied His hostess fair to greet; And others to St. Mary's went To get a bite to eat. Bold Ludwig Koch from London came, That night to Annet back they came, He travelled day and night And none dared utter word Till with his gear on Mary's Quay While Ludwig sought to test his set At last he did alight. Whereon he would record. There met him many an ardent swain Alas! A heavy dew had drenched To lend a helping hand; The cable laid with care, And after lunch they gathered round, But with a will the helpers stout A keen if motley band. -

Cordell Bank Ocean Monitoring Program (CBOMP)
Cordell Bank Ocean Monitoring Program (CBOMP) Peter Pyle1, Michael Carver1, Carol Keiper3, Ben Becker2, Dan Howard1 1Cordell Bank National Marine Sanctuary, 2Point Reyes National Seashore, 3Oikonos INTRODUCTION METHODS – OCEANOGRAPHY Cordell Bank National Marine Sanctuary (CBNMS) initiated a long-term Monitoring Program in - Thermosalinograph used to record sea surface temperature (SST) and sea surface salinity January 2004. Monitoring objectives include: continuously along transect lines. - CTD casts performed at selected locations using a SEABIRD SBE 19; data processed using - Describe the planktonic and vertebrate fauna relative to oceanography SBE software and displayed using Surfer 7.0 . - Assess temporal and spatial variation in occurrence and abundance of fauna and oceanography - Simrad EK60 echosounder with single 120Khz split-beam transducer used to estimate krill - Encourage collaborators to perform integrated ancillary research from the vessel abundance. - ArcView 9.0 Geographical Information System (GIS) used to integrate backscatter, fauna, and oceanography. SSTs were interpolated from TSG data using kriging. Temperature Sigma T Salinity Depth Figure 1. Above Survey zones for whales, birds and small mammals. Figure 2. Left Research Vessel C. magister at dock Spud Point Marina Bodega Bay Figure 3. Right Observ- ers on Transect during a Figures 5-7. CTD casts for October 13, 2004. Each colored bar represents an individual CTD cast of the 7 CTD cast locations shown in Figure CBOMP cruise in CBNMS 4. Depth of each cast is shown on the Y axis. Figure 4. Location of transects and CTD casts (dark circles) within CBNMS. PRELIMINARY RESULTS - 2004 METHODS – FAUNA - Eight surveys were conducted (due to weather and mechanical problems no surveys were - Surveys are conducted once/month using standard strip transect methodology (weather and ocean conducted in Feb, May, June, July). -

Endemic Shearwaters Are Increasing in the Mediterranean in Relation to Factors That Are Closely Related to Human Activities
Global Ecology and Conservation 20 (2019) e00740 Contents lists available at ScienceDirect Global Ecology and Conservation journal homepage: http://www.elsevier.com/locate/gecco Original Research Article Endemic shearwaters are increasing in the Mediterranean in relation to factors that are closely related to human activities * Beatriz Martín a, , Alejandro Onrubia a, Miguel Ferrer b a Fundacion Migres, CIMA, ctra. N-340, Km.85, Tarifa, E-11380, Cadiz, Spain b Applied Ecology Group, Donana~ Biological Station, CSIC, Seville, Spain article info abstract Article history: The aim of this study was to estimate global population trends of abundance of two Received 20 March 2019 endemic migratory seabird species breeding in the Mediterranean Sea, Balearic and Received in revised form 22 July 2019 Scopoli's shearwaters, from migration counts at the Strait of Gibraltar. Specifically, we Accepted 31 July 2019 assessed how regional environmental conditions (i.e. sea surface temperature, chlorophyll a concentration, NAO index and fish catches), as proxies of climate change, prey availability Keywords: and human-induced mortality factors, modulate the interannual variation in shearwater Chlorophyll numbers. The change in the migratory population size of both shearwater species was Climate change fi Fisheries estimated by tting Generalized Additive Models (GAM) to the annual counts against the fi Seabird year of observation. Speci cally, we modelled daily counts of migrant shearwaters during Monitoring the post-breeding season. Contrary to current estimates at breeding colonies, coastal-land based counts of migrating birds provide evidence that Baleric and Scopoli's shearwaters have been recently increasing in the Mediterranean Sea. Our results highlight that de- mographic patterns in these species are complex and non-linear, suggesting that most of the increases have happened recently and intimately bounded to environmental factors, such as chlorophyll concentration and fisheries, that are closely related to human activ- ities. -

ECOS 37-2-60 Reintroductions and Releases on the Isle Of
ECOS 37(2) 2016 ECOS 37(2) 2016 Reintroductions and releases on the Isle of Man Lessons from recent retreats Recent proposals for the release of white-tailed sea eagles and red squirrels on the Isle of Man received very different treatment, perhaps reflecting public perception of the animals and the public profile of the proponents, but also the political landscape of the island. NICK PINDER The Manx legal context The Isle of Man is a Crown dependency outside the EU but inside a common customs union with the United Kingdom. The Island can request that Westminster’s laws are extended to it but usually the Island passes its own laws which it promulgates at the annual Tynwald ceremony. Since it has a special relationship with the European Union, under Protocol 3, EU legislation covering agricultural and other trade is Point of Ayre: The Ayres is a large area of coastal heath and dune grassland in the north of the Isle of Mann usually translated into Manx law, as is UK law affecting customs controls. The 1980 island and location of the only National Nature Reserve. Endangered Species Act was therefore swiftly adopted in the Isle of Man but the Photo: Nick Pinder Wildlife and Countryside Act of the same year was not. A test for the legislation came in the early 1990s when some fox carcases turned When I arrived on the Isle of Man in 1987, the only wildlife legislation was a up. One was allegedly run over and then someone came forward having shot two dated Protection of Birds Act (1932-1975) but the newly formed Department of adults at a den site and dug up several cubs. -

Post-Release Survival of Fallout Newell's Shearwater Fledglings From
Vol. 43: 39–50, 2020 ENDANGERED SPECIES RESEARCH Published September 3 https://doi.org/10.3354/esr01051 Endang Species Res OPEN ACCESS Post-release survival of fallout Newell’s shearwater fledglings from a rescue and rehabilitation program on Kaua‘i, Hawai‘i André F. Raine1,*, Tracy Anderson2, Megan Vynne1, Scott Driskill1, Helen Raine3, Josh Adams4 1Kaua‘i Endangered Seabird Recovery Project (KESRP), Pacific Cooperative Studies Unit (PCSU), University of Hawai‘i and Division of Forestry and Wildlife, State of Hawai‘i Department of Land and Natural Resources, Hanapepe, HI 96716, USA 2Save Our Shearwaters, Humane Society, 3-825 Kaumualii Hwy, Lihue, HI 96766, USA 3Archipelago Research and Conservation, 3861 Ulu Alii Street, Kalaheo, HI 96741, USA 4U.S. Geological Survey, Western Ecological Research Center, Santa Cruz Field Station 2885 Mission St., Santa Cruz, CA 95060, USA ABSTRACT: Light attraction impacts nocturnally active fledgling seabirds worldwide and is a par- ticularly acute problem on Kaua‘i (the northern-most island in the main Hawaiian Island archipel- ago) for the Critically Endangered Newell’s shearwater Puffinus newelli. The Save Our Shearwaters (SOS) program was created in 1979 to address this issue and to date has recovered and released to sea more than 30 500 fledglings. Although the value of the program for animal welfare is clear, as birds cannot simply be left to die, no evaluation exists to inform post-release survival. We used satellite transmitters to track 38 fledglings released by SOS and compared their survival rates (assessed by tag transmission duration) to those of 12 chicks that fledged naturally from the moun- tains of Kaua‘i. -
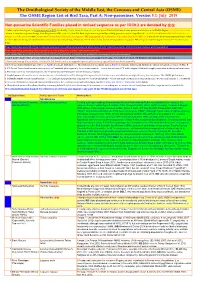
ORL 5.1 Non-Passerines Final Draft01a.Xlsx
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part A: Non-passerines. Version 5.1: July 2019 Non-passerine Scientific Families placed in revised sequence as per IOC9.2 are denoted by ֍֍ A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. English names shaded thus are taxa on BirdLife Tracking Database, http://seabirdtracking.org/mapper/index.php. Nos tracked are small. NB BirdLife still lump many seabird taxa. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. -

Status and Occurrence of Manx Shearwater (Puffinus Puffinus) in British Columbia
Status and Occurrence of Manx Shearwater (Puffinus puffinus) in British Columbia. By Rick Toochin and Louis Haviland. Introduction and Distribution The Manx Shearwater (Puffinus puffinus) is a small species of shearwater found year round along both sides of the North Atlantic Ocean (Onley and Scofield 2007). This species has breeding colonies in Iceland, France, Faeroe Island, Ireland, Scotland, England, Channel Islands, Azores Islands, Madeira Island, and the Canary Islands (Onley and Scofield 2007). In eastern North America, the Manx Shearwater breeds in Newfoundland and is found in the Gulf of St. Lawrence to the Gulf of Maine (Lee and Haney 1996). Some of these birds winter in the North Atlantic Ocean off North America from the Carolinas to Florida, but most birds are trans- equatorial migrants from July to March wintering in the South Atlantic Ocean off Brazil to Argentina and spread across the South Atlantic to South Africa (Lee and Haney 1996, Onley and Scofield 2007). The Manx Shearwater has been undergoing a somewhat mysterious range expansion over the past few decades with more and more birds turning up in the North Pacific Ocean (Roberson 1996, Mlodinow 2004). Though breeding is suspected it has yet to be proven (Mlodinow 2004). One bird was tape recorded at night in a nesting burrow on Triangle Island by Dr. Ian Jones in the summer of 1994 (Mlodinow 2004). The bird was never actually seen and the bird’s identity was left unidentified for many years (P. Jones Pers. Comm.). Another possible breeding record comes from Alaska on Middleton Island on May 12, 2005, when 2 birds were seen together possibly prospecting for a nest site (Gibson et al. -

The Status and Distribution of European Storm-Petrels Hydrobates Pelagicus and Manx Shearwaters Puffinus Puffinus on the Isles of Scilly
2002 Storm-petrels andManx Sheam aters onScilly 1 The Status and distribution of European Storm-petrels Hydrobates pelagicus and Manx Shearwaters Puffinus puffinus on the Isles of Scilly 1*, 3 4 5 V. Heaney N. Ratcliffe A. Brown P.J. Robinson & L. Lock 1, , Heaney V., Ratcliffe N., Brown A., Robinson P & Lock L. 2002. The status and distribution of European Storm-petrels Hydrobatespelagicus and Manx Shearwaters Puffinuspuffinus Seabirds This describes the on the Isles of Scilly. Atlantic 4(1): 1-16. paper first the distribution and abundance Storm- comprehensive survey of ofbreeding European Manx petrels and Shearwaters on the Isles ofScilly. Diurnal tapeplayback ofvocalisations in was used to survey those islands the archipelago on which birds had previously been reportedbreedingand to search others with suitable habitat. The total breedingpopulation ofStorm-petrels was 1475 Apparently Occupied Sites and of Manx Sheanvaters 201 Apparently OccupiedBurrows. These numbers are ofregionalimportancefor both species and the numbers of Storm-petrels are internationally important. Storm-petrel breeding distribution was restricted to rat-free outer islands, but some Manx Shearwater colonies werefoundon islands with rats and alsoferal cats. The role oferadication and control of mammalian predators in the conservation ofpetrels on the ScillyIsles is discussed. 'The Royal Society for the Protection of Birds, The Lodge, Sandy, Bedfordshire SG19 2DL, England, U.K.; "English Nature, Northminster House, Northminster Road, Peterborough, Cambridgeshire PEI 1UA, England, U.K.; ’Riviera House, Parade, St. Mary’s, Isles of 4 Scilly TR21 OLP, England, LUC.; The Royal Society for the Protection of Birds, Keble House, Southemhay Gardens, Exeter, Devon EX1 1NT, England, UK. -

Tracking, Feather Moult and Stable Isotopes Reveal Foraging Behaviour
Diversity and Distributions, (Diversity Distrib.) (2017) 23, 130–145 BIODIVERSITY Tracking, feather moult and stable RESEARCH isotopes reveal foraging behaviour of a critically endangered seabird during the non-breeding season Rhiannon E. Meier1*, Stephen C. Votier2, Russell B. Wynn1, Tim Guilford3, Miguel McMinn Grive4, Ana Rodrıguez5, Jason Newton6, Louise Maurice7, Tiphaine Chouvelon8,9, Aurelie Dessier8 and Clive N. Trueman10 1National Oceanography Centre, European ABSTRACT Way, Southampton SO14 3ZH, UK, Aim The movement patterns of marine top predators are likely to reflect 2Environment and Sustainability Institute, responses to prey distributions, which themselves can be influenced by factors University of Exeter, Penryn Campus, Penryn, Cornwall TR10 9FE, UK, 3Animal such as climate and fisheries. The critically endangered Balearic shearwater Behaviour Research Group, Department of Puffinus mauretanicus has shown a recent northwards shift in non-breeding dis- Zoology, University of Oxford, The tribution, tentatively linked to changing forage fish distribution and/or fisheries A Journal of Conservation Biogeography Tinbergen Building, South Parks Road, activity. Here, we provide the first information on the foraging ecology of this Oxford OX1 3PS, UK, 4Biogeografia, species during the non-breeding period. geodinamica i sedimentaciodela Location Breeding grounds in Mallorca, Spain, and non-breeding areas in the Mediterrania occidental (BIOGEOMED), north-east Atlantic and western Mediterranean. Universitat de les Illes Balears, Cra. de Valldemossa, km 7.5, 07122 Palma, Illes Methods Birdborne geolocation was used to identify non-breeding grounds. Balears, Spain, 5Balearic Shearwater Information on feather moult (from digital images) and stable isotopes (of Conservation Association, Puig del Teide 4 - both primary wing feathers and potential prey items) was combined to infer 315, Palmanova 07181, Illes Balears, Spain, foraging behaviour during the non-breeding season. -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose -

What Genetics Tell Us About the Conservation of the Critically Endangered Balearic Shearwater?
BIOLOGICAL CONSERVATION 137 (2007) 283– 293 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/biocon What genetics tell us about the conservation of the critically endangered Balearic Shearwater? Meritxell Genovarta,b,*, Daniel Oroa, Javier Justec, Giorgio Bertorelleb aInstitut Mediterrani d’Estudis Avanc¸ats IMEDEA (CSIC-UIB), Miquel Marque`s 21, 07190 Esporles, Mallorca, Spain bDipartimento di Biologia, Universita` di Ferrara, Via Borsari 46, 44100 Ferrara, Italy cEstacio´n Biolo´gica de Don˜ ana (CSIC), Avda. Ma Luisa s/n, Aptdo. 41080 Sevilla, Spain ARTICLE INFO ABSTRACT Article history: The Balearic shearwater Puffinus mauretanicus is one of the most critically endangered sea- Received 20 March 2006 birds in the world. The species is endemic to the Balearic archipelago, and conservation Received in revised form concerns are the low number of breeding pairs, the low adult survival, and the possible 5 February 2007 hybridization with a sibling species, the morphologically smaller Yelkouan shearwater (P. Accepted 18 February 2007 yelkouan). We sampled almost the entire breeding range of the species and analyzed the genetic variation at two mitochondrial DNA regions. No genetic evidence of population decline was found. Despite the observed philopatry, we detected a weak population struc- Keywords: ture mainly due to connectivity among colonies higher than expected, but also to a Pleis- Conservation genetics tocene demographic expansion. Some colonies showed a high imbalance between Introgression immigration and emigration rates, suggesting spatial heterogeneity in patch quality. Population expansion Genetic evidence of maternal introgression from the sibling species was reinforced, but Population structure almost only in a peripheral colony and not followed, at least to date, by the spread of the Puffinus mauretanicus introgressed mtDNA lineages.