A Statistical Approach to Multivariate Analysis of Drinking Water Quality in Kamrup District, Assam, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LIST of ACCEPTED CANDIDATES APPLIED for the POST of GD. IV of AMALGAMATED ESTABLISHMENT of DEPUTY COMMISSIONER's, LAKHIMPUR
LIST OF ACCEPTED CANDIDATES APPLIED FOR THE POST OF GD. IV OF AMALGAMATED ESTABLISHMENT OF DEPUTY COMMISSIONER's, LAKHIMPUR Date of form Sl Post Registration No Candidate Name Father's Name Present Address Mobile No Date of Birth Submission 1 Grade IV 101321 RATUL BORAH NAREN BORAH VILL:-BORPATHAR NO-1,NARAYANPUR,GOSAIBARI,LAKHIMPUR,Assam,787033 6000682491 30-09-1978 18-11-2020 2 Grade IV 101739 YASHMINA HUSSAIN MUZIBUL HUSSAIN WARD NO-14, TOWN BANTOW,NORTH LAKHIMPUR,KHELMATI,LAKHIMPUR,ASSAM,787031 6002014868 08-07-1997 01-12-2020 3 Grade IV 102050 RAHUL LAMA BIKASH LAMA 191,VILL NO 2 DOLABARI,KALIABHOMORA,SONITPUR,ASSAM,784001 9678122171 01-10-1999 26-11-2020 4 Grade IV 102187 NIRUPAM NATH NIDHU BHUSAN NATH 98,MONTALI,MAHISHASAN,KARIMGANJ,ASSAM,788781 9854532604 03-01-2000 29-11-2020 5 Grade IV 102253 LAKHYA JYOTI HAZARIKA JATIN HAZARIKA NH-15,BRAHMAJAN,BRAHMAJAN,BISWANATH,ASSAM,784172 8638045134 26-10-1991 06-12-2020 6 Grade IV 102458 NABAJIT SAIKIA LATE CENIRAM SAIKIA PANIGAON,PANIGAON,PANIGAON,LAKHIMPUR,ASSAM,787052 9127451770 31-12-1994 07-12-2020 7 Grade IV 102516 BABY MISSONG TANKESWAR MISSONG KAITONG,KAITONG ,KAITONG,DHEMAJI,ASSAM,787058 6001247428 04-10-2001 05-12-2020 8 Grade IV 103091 MADHYA MONI SAIKIA BOLURAM SAIKIA Near Gosaipukhuri Namghor,Gosaipukhuri,Adi alengi,Lakhimpur,Assam,787054 8011440485 01-01-1987 07-12-2020 9 Grade IV 103220 JAHAN IDRISH AHMED MUKSHED ALI HAZARIKA K B ROAD,KHUTAKATIA,JAPISAJIA,LAKHIMPUR,ASSAM,787031 7002409259 01-01-1988 01-12-2020 10 Grade IV 103270 NIHARIKA KALITA ARABINDA KALITA 006,GUWAHATI,KAHILIPARA,KAMRUP -

Prospectus-2019.Pdf
TEZPUR UNIVERSITY PROSPECTUS Autumn 2019 Tezpur University Napaam, Sonitpur Assam, INDIA 784028 www.tezu.ernet.in Contents Section I General Information about the University…………..…………… 3-16 Section II Programmes, Intake and Eligibility for Admission……………. 17-29 Section III Admission Procedure……………………………………………………… 30-38 Section IV Departments/ Centres………………………………………………………. 39-165 Section V Important Dates, Fee Structure, Forms and Contact Details… 166-178 SECTION- I General Information about the University 1.1 Introduction 1.2 Awards/Accolades 1.3 Facilities and Services 1.4 Training and Placement Cell 1.5 List of Academic Programmes 1.6 Curricula 1.7 Evaluation System 1.8 Important academic Rules 1.9 Important Rules GENERAL INFORMATION ABOUT THE UNIVERSITY 1.1 : Introduction Tezpur University was established on January 21, 1994 by an Act of Parliament of India, The Tezpur University Act, 1993 (Act No. 45 of 1993), as a non-affiliating and residential Central University. The University is located at Napaam, about 15 km east of Tezpur town in the Sonitpur District of Assam. The serene and green University Campus of about 262 acres provides an excellent ambience including modern infrastructure conducive for learning and dedicated research. The academic programmes, offered in the University, have a distinct focus on Science, Technology, Management, Humanities, and Social Sciences, reflecting the objectives of the University. At present, the University offers a number of Programmes of Under-Graduate Degree/Diploma/Certificate, Integrated Programmes, Post-Graduate Degree/Diploma and Doctor of Philosophy Degree in various Disciplines. The University offers Add-on courses on Yoga and Violin too. During the last 25 years of its existence, the University has engaged itself in the process of capacity building, both in terms of infrastructure and human resource development. -

My-Profile 1 Ms. Bashabi Gogoi MA (Tezpur University)
My-Profile Bashabi Gogoi MA (Tezpur University), PhD (Gauhati University), MPhil (EFL University, Shillong) th Date of Joining: Aug 26 ,2011 Mobile: 8011791942 Email: [email protected] Areas of Interest: Indian Writing in English, Film Studies and Adaptation theories. Publications: Gogoi, Bashabi. Spinning a Fantastic Yarn: Reading Vikram Chandra's Red Earth and Pouring Rain as a Postmodern Narrative. Assonance: A Journal of Russian and Comparative Literary Studies. No 21. Jan 2021. ISSN 2394-7853, pp 91-100. Print. Gogoi, Bashabi and Das, Kandarpa. Calm Without and Fire Within: Adapting the Devdas Metaphor for the Screen. Assonance: A Journal of Russian and Comparative Literary Studies. No 19. Jan 2019. ISSN 2394-7853, pp 63-83. Print. Gogoi, Bashabi and Kandarpa Das. "Love in the Time of Revolution: A Study of the Novel and Film Adaptation of Boris Pasternak's Doctor Zhivago". Assonance: A Journal of Russian and Comparative Literary Studies 16 (2016): 98-115. Print. Gogoi, Bashabi. "Striving for Grace: A Study of the Novel and Film Adaptation of J. M. Coetzee’s Disgrace". Adaptations: Some Journeys from Words to Visuals. Eds. Shri Krishan Rai and Anugamini Rai. Newcastle: Cambridge Scholars Publishing, 2015. 142-154. Print. Gogoi, Bashabi. “Postmodern Indian Fiction in English: Narrative Strategy in Vikram Chandra’s Love and Longing in Bombay”. Postmodern Indian Fiction in English: Some Perspectives. Ed. Abha Shukla Kaushik. Jaipur: Aadi Publications, 2012. 78-98. Print. Papers presented in National/ International Conferences: “Explorations and Consolidation of Colonial Power: A Reading of Major John Butler’s Travels and Adventures in the Province of Assam (During a Residence of Fourteen Years)”. -

Assam Science and Technology University
ASSAM SCIENCE AND TECHNOLOGY UNIVERSITY (A State University of Government of Assam constituted by “Assam Science and Technology University Act, 2009”) Tetelia Road, Jalukbari, Guwahatiiah -781013, Assam NOTICE Due to some technical problem, the registered participants for NCRAST 2018 are requested to send your abstract and contact details (email address, mobile number, Institute address) with the on-line payment receipt once again to [email protected] or before 6th March, 2018. The list of the registered participants is listed below: Sl. Name Institute Address No. 1 Dharitree Dutta Gauhati University Gauhati University Campus, Ghy-14 2 BijitNandan Sharma Royal Group Of Insitution Betkuchi 3 N. Hemarjit Singh NERIST Khanamukh 4 Shamsuz Zaman JEC Jorhat-07 5 Prahlad Kumar Baruah IITG North Guwahati 6 Pallabi Pathak IASST PaschimBoragaon, ghy 7 PalashJyoti Boruah IASST PaschimBoragaon, ghy 8 BijoyPatir Gauhati University Guwahati 9 Yoshiko Bailung IASST Ghy-35 10 HimangshuDeka GIPS Azara 11 Devi Violina GIPS Azara 12 RinkuDeka Gauhati University Guwahati 13 ShahinaKhanam Chowdhury GIPS Azara 14 Nazneen Dewan GIPS Azara 15 TejendraSaikia GIPS Azara 16 AnkitaSarma GIPS Azara 17 AzmolHoque GIPS Azara 18 SunitiChangmai Gauhati University Guwahati 19 Anusmita Das Gauhati University Guwahati 20 SamiranGoswami GIMT Guwahati 21 NaishargaKollol GIMT Guwahati 22 Gongotri Hazarika Kaliabor College Kaliabor 23 MaisnamBabita Devi Assam Don Bosco University Azara 24 Sagorika Bora Kaliabor College Kaliabor 25 Bipul Kumar Talukdar Gauhati University -

International Journal of History & Scientific Approach
International Journal of History & Scientific Approach History and Culture: A Study on the Importance of the Place Names of Chandrapur Rosie Patangia Assistant Professor and Head, Deptt of English, Narangi Anchalik Mahavidyalaya, Narengi, Guwahati-171, Dist: Kamrup (Metro), Assam, India & Ph.D Research Scholar, Folklore Department, Gauhati University, Guwahati- 781014, Assam, India Abstract: History has its culture. Every place is denoted by a name. Each place name carries its own culture and tradition. The memory of a place is deeply embedded in its history, historical characters, legendary heroes, historical events etc. Assam in general and Kamrup in particular is rich in its culture and history. The place names speak of the past history of that place and helps in building identity. In Assam, we find innumerable places connected with oral narratives, folk beliefs, culture, history, myths, and legends. The present paper is an attempt to study the place names of Chandrapur and its cultural and historical significance. Key words: History, Culture, Place, Names, Chandrapur, historical significant. Introduction Every place from the macrocosm to the microcosm embodies its community, history and culture. Robert Murphy (1986) highlights that “Culture means the total body of tradition borne by a society and transmitted from generation to generation.” The Oxford English Dictionary (2007) defines a ‘place’ as a ‘particular position or area or a portion of space occupied by or set aside for someone or something’. It defines a ‘name’ as ‘a word or words by which someone or something is known.”Each place has its own cultural and historical background. While the term ‘cultural’ refers to the ideas, customs and social behavior of a society, the word ‘historical’ relates to past events or history. -
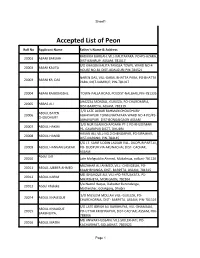
Accepted List of Peon
Sheet1 Accepted List of Peon Roll No Applicant Name Father's Name & Address RADHIKA BARUAH, VILL-KALITAPARA. PO+PS-AZARA, 20001 ABANI BARUAH DIST-KAMRUP, ASSAM, 781017 S/O KHAGEN KALITA TANGLA TOWN, WARD NO-4 20002 ABANI KALITA HOUSE NO-81 DIST-UDALGURI PIN-784521 NAREN DAS, VILL-GARAL BHATTA PARA, PO-BHATTA 20003 ABANI KR. DAS PARA, DIST-KAMRUP, PIN-781017 20004 ABANI RAJBONGSHI, TOWN-PALLA ROAD, PO/DIST-NALBARI, PIN-781335 AHAZZAL MONDAL, GUILEZA, PO-CHARCHARIA, 20005 ABBAS ALI DIST-BARPETA, ASSAM, 781319 S/O LATE AJIBAR RAHMAN CHOUDHURY ABDUL BATEN 20006 ABHAYAPURI TOWN,NAYAPARA WARD NO-4 PO/PS- CHOUDHURY ABHAYAPURI DIST-BONGAIGAON ASSAM S/O NUR ISLAM CHAPGARH PT-1 PO-KHUDIMARI 20007 ABDUL HAKIM PS- GAURIPUR DISTT- DHUBRI HASAN ALI, VILL-NO.2 CHENGAPAR, PO-SIPAJHAR, 20008 ABDUL HAMID DIST-DARANG, PIN-784145 S/O LT. SARIF UDDIN LASKAR VILL- DUDPUR PART-III, 20009 ABDUL HANNAN LASKAR PO- DUDPUR VIA ARUNACHAL DIST- CACHAR, ASSAM Abdul Jalil 20010 Late Mafiguddin Ahmed, Mukalmua, nalbari-781126 MUZAHAR ALI AHMED, VILL- CHENGELIA, PO- 20011 ABDUL JUBBER AHMED KALAHBHANGA, DIST- BARPETA, ASSAM, 781315 MD ISHAHQUE ALI, VILL+PO-PATUAKATA, PS- 20012 ABDUL KARIM MIKIRBHETA, MORIGAON, 782104 S/o Nazrul Haque, Dabotter Barundanga, 20013 Abdul Khaleke Motherjhar, Golakgonj, Dhubri S/O MUSLEM MOLLAH VILL- GUILEZA, PO- 20014 ABDUL KHALEQUE CHARCHORRIA, DIST- BARPETA, ASSAM, PIN-781319 S/O LATE IDRISH ALI BARBHUIYA, VILL-DHAMALIA, ABDUL KHALIQUE 20015 PO-UTTAR KRISHNAPUR, DIST-CACHAR, ASSAM, PIN- BARBHUIYA, 788006 MD ANWAR HUSSAIN, VILL-SIOLEKHATI, PO- 20016 ABDUL MATIN KACHARIHAT, GOLAGHAT, 7865621 Page 1 Sheet1 KASHEM ULLA, VILL-SINDURAI PART II, PO-BELGURI, 20017 ABDUL MONNAF ALI PS-GOLAKGANJ, DIST-DHUBRI, 783334 S/O LATE ABDUL WAHAB VILL-BHATIPARA 20018 ABDUL MOZID PO&PS&DIST-GOALPARA ASSAM PIN-783101 ABDUL ROUF,VILL-GANDHINAGAR, PO+DIST- 20019 ABDUL RAHIZ BARPETA, 781301 Late Fizur Rahman Choudhury, vill- badripur, PO- 20020 Abdul Rashid choudhary Badripur, Pin-788009, Dist- Silchar MD. -

North-East Summar Workshop in Analysis and Probability-2016 List
North-East Summar Workshop in Analysis and Probability-2016 List of selected Male Participants for the workshop with accommodation Name of the Participant S. No Address Accommodation at Mr. Muhim Chutia Department of Mathematics Chandini Hotel Nahalagun 1 Assistant Professor opposite of Nahalagun Moriani College, Assam Stedium Mr. Ranjan Kumar Gogoi Department of Mathematics 2 Associate Professor Do Tinsukia College,Tinsukia, Assam Mr. Birinchi Kumar Boruah Department of Mathematics Do 3 Ph.D Scholar, Tezpur University, Assam Dr. Tapan Kumar Baishya Department of Mathematics Do 4 Assistant Professor D.R.College, Golaghat, Assam Mr. Pranjal Borah Department of Mathematics Do 5 Assistant Professor D.R.College, Golaghat, Assam Mr. Splendour Rein S. Sawkmie Department of Mathematics Do 6 Assistant Professor St. Mary’s College, Shillong Dr. Bharat Borah Department of Mathematics Do 7 Assistant Professor St. Mary’s College, Shillong Mr. Laba Handique Department of Statistics Do 8 Research Scholar Dibrugarh University, Assam Mr. Manash Pratim Kashyap Department of Statistics Chandini Hotel Nahalagun 9 Assam Down Town University, Guwahati opposite of Nahalagun Stedium 10 Mr. Kuldeep Sarma Tezpur University M.Sc Pass student Do Gopal Bazar, Nalbari Assam 11 Mr. Ajay Sharma Department of Mathematics Do Tezpur University, Ph.D Scholar 12 Dr. Rupam Kr. Gogoi Department of Mathematics Do Associate Professor Sibsagar College, Joysagar 13 Mr. Bidyut Baruah Department of Mathematics Do Asssistant Professor, Sibsagar Girls College 14 Mr. Nava Jyoti Hazarika Department of Mathematics Do Assistant Professor Tyagbir Hem Baruah College, Jamaguri, Sunitpur 15 Mr. Bhaskar Basumatari Department of Mathematics Do Assistant Professor Sibsagar College, Joysagar, Assam 16 Mr. -

Gauhati University Gopinath Bardoloi Nagar::Guwahati- 14, Assam
,0n+'t'f 9/N( flJa,ry ,,Aw*l"ru,Xf , (*orpao 4 'u/* -t) tf ?a GAUHATI UNIVERSITY GOPINATH BARDOLOI NAGAR: :GUWAHATI. 14, ASSAM :: INDIA NorrFrcArroN -?V0'6S' ' It is notified for information of all concerned that the Academic Council, G.U. in its meeting held on 29/061201& has approved the CCS (UG) for the Department of Economics, GU with the following members for a period of 3 (three) years w.e.f. the date of notification. This is issued with the approval of the Hon'ble Vice-Chancellor, G.U. dated 1410812018. 1. Archana Sharma, Professor, Department of Economics, GU 2. Nissar A. Baruah, Professor, Department of Economics, GU 3. Gayatri Goswami, Associate Professor, Department of Economics, GU 4. Nivedita Goswami, Associate Professor, Deparfinent of Economics, GU 5. Jagadish Sarma, Department of Economics, Barnagar College 6, Mitali Das, Department of Economics, Cotton University ,,t ltaryChaudhury, Department of Economics, Pragiyotish College 8. Nandita Goswami, Department of Ec-onomics, Baibhag College ,9. Bhupen Kumar Sarma, Department of Economics, Jagiroad College 10. M.R. Kazi, Department of Economics, Mankachar College 11. Angana Barua, Department of Economics, Pandu College 12.14ousumi Borah, Department of Economics, Arya Vidyapeeth College 13. Mahendra Hazarika, Department of Economics, Chaiduar College 14. Mausumi Kalita, Department of Economics, Kharupetia College 15. M.P. Bezbaruah, Department of Economics, GU (Ex Oflicio Convener) Sd/- Dr. G. Khataniar Academic Registrar Gauhati University Memo No. GU/NI/AR/ccs/201 st 60l I - 6013 Date: A|ls Copy forwarded for information and necessary action to:- ll'- l. Members concerned. 2.' Dean, Faculty of Arts, G.U. -

E4182 V1: Draft Final Report Vol. I
Public Disclosure Authorized Consultancy Services for Undertaking Environmental Assessment for the Rural Water Supply & Sanitation Project in Assam Public Disclosure Authorized March 2013 DRAFT FINAL REPORT VOLUME I Public Disclosure Authorized Submitted To: Chief Engineer (PHE), Assam. World Bank Project, Hengrabari,Guwahati-781036 Submitted By: IPE Global Pvt. Ltd. Public Disclosure Authorized (Formerly Infrastructure Professionals Enterprise (P) Ltd.) Address: IPE Towers, B-84, Defence Colony, Bhisham Pitamah Marg, New Delhi – 110024, India Tel: +91-11-40755920, 40755923; Fax: +91-11-24339534 Consultancy Services for Undertaking Environmental Assessment for the Rural Water Supply & Sanitation Project in Assam Draft Final Report Table of Contents Abbreviations and Acronyms ...............................................................................................................9 Executive Summary .......................................................................................................................... 11 1. INTRODUCTION ......................................................................................................................... 21 1.1 Background ....................................................................................................................... 21 1.2 Present World Bank Assisted Rural Water Supply and Sanitation Project............................... 23 1.2.1 Components............................................................................................................... 23 -
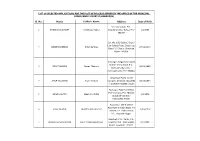
List of Selected Applications for the Post of Process Server in the Office of the Principal Judge,Family Court-Ii,Kamrup(M)
LIST OF SELECTED APPLICATIONS FOR THE POST OF PROCESS SERVER IN THE OFFICE OF THE PRINCIPAL JUDGE,FAMILY COURT-II,KAMRUP(M) Sl. No. Name Father's Name Address Date of Birth Vill- Charaimari, P.O.- 1 SANJIB CHOUDHURY Dimbeswar Kalita Charaimari, Dist- Baksa, Pin.- 1/1/1988 781377 Qrt. No. 102, Block-C, Dispur Law College Road, Dispur Last 2 BROJEN BARMAN Girish Barman 27/11/1994 Gate, P.S.- Dispur, Guwahati, Assam-781006. Gitanagar, Batgastall,Giripath Mother teresa Road, P.O.- 3 NITU THAKURIA Deben Thakuria 26/11/1986 Bamunimaidan, Dist.- Kamrup(Assam), Pin- 781021. Hengrabari Public Health 4 ANUP TALUKDAR Aswini Talukdar Complex, (H) block, House No- 13/12/1994 4, Guwahati-781036, Assam Rupnagar, Pialy Path-M-20, P.O.- Indrapur, Pin- 781032, 5 BRAJEN DUTTA Manik Ch. Dutta 1/5/1975 Guwahati- 32, Dist.- Kamrup(M) Assam. House No.- 154 B, GMCH Road Near Birubari Bazar, Pin- 6 FIROZ AHMED Mukhtaruddin Ahmed 25/06/1993 781016, P.S.- Paltan Bazar, P.S.- Gopinath Nagar. Guwahati, P.O.- Pandu, P.S.- 7 HRISHIKESH UPADHYAYA Khem Prasad Upadhyaya Jalukbari, Dist.- Kamrup(M), 4/1/1988 Assam. Guwahati.-781012. LIST OF SELECTED APPLICATIONS FOR THE POST OF PROCESS SERVER IN THE OFFICE OF THE PRINCIPAL JUDGE,FAMILY COURT-II,KAMRUP(M) Vill- Gadhoputa, P.O.- Rajapara, P.S.- Boko, Dist.- 8 SAMIRAN RABHA Subhash Rabha 27/12/1981 Kamrup® Assam, Pin.- 781135 Vill- B.B. Road Bazar, Barpeta, 9 NILOTPAL DAS Dipak Kumar Das P.O.- Barpeta, Dist.- 18/02/1991 Barpeta,Assam, Pin-781301. Vill- Gabhara, P.S.- Sipajhar,P.o- 10 UTPAL CHAMUA Nandeswar Chamua Duni, Dist.- Darrang, Pin- 30/06/1988 784148. -

Village & Town Directory, Darrang, Part-XIII-A, Series-3, Assam
CENSllS OF INDIA 1981 SElUES-3 : ·ASSAM DISTRICT CENSUS HANDBOOK J>~1f "III-A VILLAGE & TOWN DIRECTORY DARRANG DISTRICT N. K. CHOUDHURY of the Indian Administrative Service DIRECTOR OF CENSUS OPERATIONS ASSAM Printed at THE BHARAT PRESS Santipur * Guwahati-781 009 CONTENTS Pages Foreword 1-11 Preface III Analytical Note. v-xxm Section- J: Village Directory 1 - 411 Note explaining the codes used in the village Directory (1-3), Paneri P.S -Alphabetical list of villages (4-7), Amenities and land use (8-41), Udalguri P.S. -Alphabetical list of villages (42-44), Amenities and land use (46-65), Maz.bat P .8.- Alpha betical list of villages (66-67), Amenities and land use (68-85), Kalaigaon P.S. - Alphabetical list of villages (86-89), Ameni ties and land use (9)-115), Dalgaon P.S. - Alphabetrcal list of villages (116-119), AOJeni ties and land use (120-143), Mangal doi P.S. - Alphabetical list of villages (144-149), Amenities and land use (150-191), Dhekiajuli P.S. - Alphabetical list of villages (192-197), Amenities and land use (198-243), Tezpur P.S. - Alphabetical list of villages (244-247), Ameni ties and land use (248-273), Rangapara P.S. - Alpbabetical list of villages (274-275), Amenities and land use (276-293), Chutia P.S. - AlphabeticalJistof villages (294-299), Amenities and land use (300-343), .BehaH P .S. X~habetjcal list of villages (344-347), Am~nities apd ll\.n.<! .u~e. (348~377), Gohpur P.S. - Alphabetical li~t of villages (378-"381), Amenities and land use (382-411). Appendix I Police Station wise Abstract of Educational, Medical and other 413-416 amenities. -

Gauhati University Gopinath Bardoloi Nagar Guwahati-781014, Assam (India)
GAUHATI UNIVERSITY GOPINATH BARDOLOI NAGAR GUWAHATI-781014, ASSAM (INDIA) Ref.No: SECY/UC/2019/3259 Date: 15/10/2019 NOTIFICATION The following first semester Post Graduate Female Students who have applied for hostel accommodation vide notification no. SECY/UC/2019/2987 dated 18-09-2019 have been selected against the vacant seats at various G.U. Hostels as given below: Sl. Application Name Department/ Programme Allotted Hostel No. ID 1 2021927 Suriya Nunisa Anthropology RCC-7 Girl's Hall 2 2024151 Aeruja Begum Arabic RCC-7 Girl's Hall 3 2028288 Salina Jaklina Parbin Assamese AT-3 Girl's Hall 4 2022538 Gitika Bora Assamese RCC-2 Girl's Hall 5 2023884 Jurijyoti Ray Assamese RCC-3 Girl's Hall 6 2030693 Rajeshree Gogoi Assamese RCC-4 Girl's Hall 7 2021501 Chitrali Chetia Assamese RCC-5 Girl's Hall 8 2019346 Karishma Phukan Assamese RCC-7 Girl's Hall 9 2030397 Shahanas Aktara Assamese RCC-1 Girl's Hall 10 2024218 Anasmita Patgiri Assamese RCC-6 Girl's Hall 11 2021846 Farhana Wahid Assamese RCC-7 Girl's Hall 12 2020440 Bandita Gogoi Assamese RCC-5 Girl's Hall 13 2028889 Krishangi Barman Assamese RCC-6 Girl's Hall 14 2029231 Farhin Ahmed Botany RCC-4 Girl's Hall 15 2024531 Trishna Jarambasa Botany RCC-5 Girl's Hall 16 2029449 Minakshi Gohain Chemistry RCC-4 Girl's Hall 17 2034046 Jimismita Barman Comparative Indian Literature RCC-5 Girl's Hall 18 2027986 Anju Hazarika Communication and Journalism AT-3 Girl's Hall 19 2028244 Bhupali Tamuli Communication and Journalism RCC-2 Girl's Hall 20 2030816 Bijoylakshmi Borah Communication and Journalism