Diethylstilbestrol Induces Fish Oocyte Maturation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Suitability of Immobilized Systems for Microbiological Degradation of Endocrine Disrupting Compounds
molecules Review Suitability of Immobilized Systems for Microbiological Degradation of Endocrine Disrupting Compounds Danuta Wojcieszy ´nska , Ariel Marchlewicz and Urszula Guzik * Institute of Biology, Biotechnology and Environmental Protection, Faculty of Natural Science, University of Silesia in Katowice, Jagiello´nska28, 40-032 Katowice, Poland; [email protected] (D.W.); [email protected] (A.M.) * Correspondence: [email protected]; Tel.: +48-3220-095-67 Academic Editors: Urszula Guzik and Danuta Wojcieszy´nska Received: 10 September 2020; Accepted: 25 September 2020; Published: 29 September 2020 Abstract: The rising pollution of the environment with endocrine disrupting compounds has increased interest in searching for new, effective bioremediation methods. Particular attention is paid to the search for microorganisms with high degradation potential and the possibility of their use in the degradation of endocrine disrupting compounds. Increasingly, immobilized microorganisms or enzymes are used in biodegradation systems. This review presents the main sources of endocrine disrupting compounds and identifies the risks associated with their presence in the environment. The main pathways of degradation of these compounds by microorganisms are also presented. The last part is devoted to an overview of the immobilization methods used for the purposes of enabling the use of biocatalysts in environmental bioremediation. Keywords: EDCs; hormones; degradation; immobilization; microorganisms 1. Introduction The development of modern tools for the separation and identification of chemical substances has drawn attention to the so-called micropollution of the environment. Many of these contaminants belong to the emergent pollutants class. According to the Stockholm Convention they are characterized by high persistence, are transported over long distances in the environment through water, accumulate in the tissue of living organisms and can adversely affect them [1]. -

SPIRONOLACTONE Spironolactone – Oral (Common Brand Name
SPIRONOLACTONE Spironolactone – oral (common brand name: Aldactone) Uses: Spironolactone is used to treat high blood pressure. Lowering high blood pressure helps prevent strokes, heart attacks, and kidney problems. It is also used to treat swelling (edema) caused by certain conditions (e.g., congestive heart failure) by removing excess fluid and improving symptoms such as breathing problems. This medication is also used to treat low potassium levels and conditions in which the body is making too much of a natural chemical (aldosterone). Spironolactone is known as a “water pill” (potassium-sparing diuretic). Other uses: This medication has also been used to treat acne in women, female pattern hair loss, and excessive hair growth (hirsutism), especially in women with polycystic ovary disease. Side effects: Drowsiness, lightheadedness, stomach upset, diarrhea, nausea, vomiting, or headache may occur. To minimize lightheadedness, get up slowly when rising from a seated or lying position. If any of these effects persist or worsen, notify your doctor promptly. Tell your doctor immediately if any of these unlikely but serious side effects occur; dizziness, increased thirst, change in the amount of urine, mental/mood chances, unusual fatigue/weakness, muscle spasms, menstrual period changes, sexual function problems. This medication may lead to high levels of potassium, especially in patients with kidney problems. If not treated, very high potassium levels can be fatal. Tell your doctor immediately if you notice any of the following unlikely but serious side effects: slow/irregular heartbeat, muscle weakness. Precautions: Before taking spironolactone, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. -
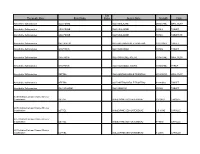
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

Postmenopausal Pharmacotherapy Newsletter
POSTMENOPAUSAL PHARMACOTHERAPY September, 1999 As Canada's baby boomers age, more and more women will face the option of Hormone Replacement Therapy (HRT). The HIGHLIGHTS decision can be a difficult one given the conflicting pros and cons. M This RxFiles examines the role and use of HRT, as well as newer Long term HRT carries several major benefits but also risks SERMS and bisphosphonates in post-menopausal (PM) patients. which should be evaluated on an individual and ongoing basis MContinuous ERT is appropriate for women without a uterus HRT MWomen with a uterus should receive progestagen (at least 12 HRT is indicated for the treatment of PM symptoms such as days per month or continuous low-dose) as part of their HRT vasomotor disturbances and urogenital atrophy, and is considered MLow-dose ERT (CEE 0.3mg) + Ca++ appears to prevent PMO primary therapy for prevention and treatment of postmenopausal MBisphosphinates (e.g. alendronate, etidronate) and raloxifene are osteoporosis (PMO).1 Contraindications are reviewed in Table 2. alternatives to HRT in treating and preventing PMO Although HRT is contraindicated in women with active breast or M"Natural" HRT regimens can be compounded but data is lacking uterine cancer, note that a prior or positive family history of these does not necessarily preclude women from receiving HRT.1 Comparative Safety: Because of differences between products, some side effects may be alleviated by switching from one product Estrogen Replacement Therapy (ERT) 2 to another, particularly from equine to plant sources or from oral to Naturally secreted estrogens include: topical (see Table 3 - Side Effects & Their Management). -

Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals
Journal of Clinical Medicine Review Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals Carlotta Cocchetti 1, Jiska Ristori 1, Alessia Romani 1, Mario Maggi 2 and Alessandra Daphne Fisher 1,* 1 Andrology, Women’s Endocrinology and Gender Incongruence Unit, Florence University Hospital, 50139 Florence, Italy; [email protected] (C.C); jiska.ristori@unifi.it (J.R.); [email protected] (A.R.) 2 Department of Experimental, Clinical and Biomedical Sciences, Careggi University Hospital, 50139 Florence, Italy; [email protected]fi.it * Correspondence: fi[email protected] Received: 16 April 2020; Accepted: 18 May 2020; Published: 26 May 2020 Abstract: Introduction: To date no standardized hormonal treatment protocols for non-binary transgender individuals have been described in the literature and there is a lack of data regarding their efficacy and safety. Objectives: To suggest possible treatment strategies for non-binary transgender individuals with non-standardized requests and to emphasize the importance of a personalized clinical approach. Methods: A narrative review of pertinent literature on gender-affirming hormonal treatment in transgender persons was performed using PubMed. Results: New hormonal treatment regimens outside those reported in current guidelines should be considered for non-binary transgender individuals, in order to improve psychological well-being and quality of life. In the present review we suggested the use of hormonal and non-hormonal compounds, which—based on their mechanism of action—could be used in these cases depending on clients’ requests. Conclusion: Requests for an individualized hormonal treatment in non-binary transgender individuals represent a future challenge for professionals managing transgender health care. For each case, clinicians should balance the benefits and risks of a personalized non-standardized treatment, actively involving the person in decisions regarding hormonal treatment. -

Exposure to Female Hormone Drugs During Pregnancy
British Journal of Cancer (1999) 80(7), 1092–1097 © 1999 Cancer Research Campaign Article no. bjoc.1998.0469 Exposure to female hormone drugs during pregnancy: effect on malformations and cancer E Hemminki, M Gissler and H Toukomaa National Research and Development Centre for Welfare and Health, Health Services Research Unit, PO Box 220, 00531 Helsinki, Finland Summary This study aimed to investigate whether the use of female sex hormone drugs during pregnancy is a risk factor for subsequent breast and other oestrogen-dependent cancers among mothers and their children and for genital malformations in the children. A retrospective cohort of 2052 hormone-drug exposed mothers, 2038 control mothers and their 4130 infants was collected from maternity centres in Helsinki from 1954 to 1963. Cancer cases were searched for in national registers through record linkage. Exposures were examined by the type of the drug (oestrogen, progestin only) and by timing (early in pregnancy, only late in pregnancy). There were no statistically significant differences between the groups with regard to mothers’ cancer, either in total or in specified hormone-dependent cancers. The total number of malformations recorded, as well as malformations of the genitals in male infants, were higher among exposed children. The number of cancers among the offspring was small and none of the differences between groups were statistically significant. The study supports the hypothesis that oestrogen or progestin drug therapy during pregnancy causes malformations among children who were exposed in utero but does not support the hypothesis that it causes cancer later in life in the mother; the power to study cancers in offspring, however, was very low. -

Estrogen Actions Throughout the Brain
Estrogen Actions Throughout the Brain BRUCE MCEWEN Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology, The Rockefeller University, New York, New York 10021 ABSTRACT Besides affecting the hypothalamus and other brain areas related to reproduction, ovarian steroids have widespread effects throughout the brain, on serotonin pathways, catecholaminergic neurons, and the basal forebrain cholinergic system as well as the hippocampal formation, a brain region involved in spatial and declarative memory. Thus, ovarian steroids have measurable effects on affective state as well as cognition, with implications for dementia. Two actions are discussed in this review; both appear to involve a combination of genomic and nongenomic actions of ovarian hormones. First, regulation of the serotonergic system appears to be linked to the presence of estrogen- and progestin-sensitive neurons in the midbrain raphe as well as possibly nongenomic actions in brain areas to which serotonin neurons project their axons. Second, ovarian hormones regulate synapse turnover in the CA1 region of the hippocampus during the 4- to 5-day estrous cycle of the female rat. Formation of new excitatory synapses is induced by estradiol and involves N-methyl-D-aspartate (NMDA) receptors, whereas downregulation of these synapses involves intracellular progestin receptors. A new, rapid method of radioimmunocytochemistry has made possible the demonstration of synapse formation by labeling and quantifying the specific synaptic and dendritic molecules involved. Although NMDA receptor activation is required for synapse formation, inhibitory interneurons may play a pivotal role as they express nuclear estrogen receptor-alpha (ER␣). It is also likely that estrogens may locally regulate events at the sites of synaptic contact in the excitatory pyramidal neurons where the synapses form. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Illinois Medicaid Preferred Drug List
Illinois Medicaid Preferred Drug List Effective January 1, 2020 (Updated January 27, 2020) ▪ The Preferred Drug List (PDL) has products listed in groups by drug class, drug name, dosage form, and PDL status ▪ Multi-source drugs are listed by both brand and generic names when applicable ▪ ADHD Agents: Prior authorization required for participants under 6 years of age and participants 19 years of age and older ▪ Spiriva AER 1.25mcg: Prior authorization NOT required for participants ages 6-17 years ▪ Budesonide: Prior authorization NOT required for participants age 7 years and under ▪ Anticonvulsants: Prior authorization NOT required for non-preferred epilepsy agents for those participants with a diagnosis of epilepsy or seizure disorder in Department records ▪ Antipsychotics: Prior authorization required for participants under 8 years of age and long-term care residents ▪ For drugs not found on this list, go to the drug search engine at: www.ilpriorauth.com Page 1 of 92 DOSAGE DRUG CLASS DRUG NAME PDL STATS FORM ADHD / ANTI-NARCOLEPSY AGENTS : AMPHETAMINES ADZENYS ER SUER NON_PREFERRED AMPHETAMINE ER SUER NON_PREFERRED DYANAVEL XR SUER NON_PREFERRED ADZENYS XR-ODT TBED NON_PREFERRED AMPHETAMINE SULFATE TABS NON_PREFERRED EVEKEO TABS NON_PREFERRED EVEKEO ODT TBDP NON_PREFERRED DEXEDRINE CP24 NON_PREFERRED DEXTROAMPHETAMINE SULFATE ER CP24 NON_PREFERRED DEXTROAMPHETAMINE SULFATE SOLN NON_PREFERRED PROCENTRA SOLN NON_PREFERRED DEXTROAMPHETAMINE SULFATE TABS NON_PREFERRED ZENZEDI TABS NON_PREFERRED VYVANSE CAPS PREFERRED VYVANSE CHEW PREFERRED -

Health Effects of Progestins Contained in Cocs, with A
A PHACTICAL JOURNAL FOR NUHSE PRACTITIONEn5 I i - 1- November 2007 The Official Journal of NPWHj- A 7 .-. Vol. G, No. 11 A Peer-Reviewed Journal &$ Health Effects of Progestins Contained in t COCs, with a -.'P Focus on Norgestimate- -.-I. 21 ? NPWH 2007 ' Annual Conference Abstracts HEALTH EFFECTS OF PROLESTINS CONTAINED IN COCs, WITH A FOGUS ON NORGESTIMATE by Penelope M. Bosarge, MSN, WHNP ased on interviews with more Nurse practitioners (NPs) and their reproductive-aged than 7600 female adolescents and women aged 15 to 44 patients face numerous challenges, particularly when it years, more than 98% who have comes to choosing a method of birth control. If a combined ever had sexual intercourse have used at least one contraceptive oral contraceptive (COC) is judged to be an appropriate meth~d.~The leading method of contraception in the United choice for a given woman, then her NP will need to select a States in 2002 was the Pill, used particular product from among many that are available. by 11.6 million women, and the second leading method was Among the various COC brands on the market, several female sterilization, used by 10.3 million women.2The condom contain norgestimate (NGM), a progestin introduced in was the third-leading method, 1992. Fvteen years of clinical use of NGM-containing used by about 9 million women and their partners. The condom COCs, as well as continued scientz$c investigations of these is the leading method at first intercourse, but the Pill is the products, support their efficacy, safety, and tolerability leading method among women (ie, these products have minimal androgenic and estrogenic younger than 30 yearse2 effects and provide excellent cycle control). -

Hormone Replacement Therapy and Osteoporosis
This report may be used, in whole or in part, as the basis for development of clinical practice guidelines and other quality enhancement tools, or a basis for reimbursement and coverage policies. AHRQ or U.S. Department of Health and Human Services endorsement of such derivative products may not be stated or implied. AHRQ is the lead Federal agency charged with supporting research designed to improve the quality of health care, reduce its cost, address patient safety and medical errors, and broaden access to essential services. AHRQ sponsors and conducts research that provides evidence-based information on health care outcomes; quality; and cost, use, and access. The information helps health care decisionmakers— patients and clinicians, health system leaders, and policymakers—make more informed decisions and improve the quality of health care services. Systematic Evidence Review Number 12 Hormone Replacement Therapy and Osteoporosis Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 2101 East Jefferson Street Rockville, MD 20852 http://www.ahrq.gov Contract No. 290-97-0018 Task Order No. 2 Technical Support of the U.S. Preventive Services Task Force Prepared by: Oregon Health Sciences University Evidence-based Practice Center, Portland, Oregon Heidi D. Nelson, MD, MPH August 2002 Preface The Agency for Healthcare Research and Quality (AHRQ) sponsors the development of Systematic Evidence Reviews (SERs) through its Evidence-based Practice Program. With guidance from the third U.S. Preventive Services Task Force∗ (USPSTF) and input from Federal partners and primary care specialty societies, two Evidence-based Practice Centers—one at the Oregon Health Sciences University and the other at Research Triangle Institute-University of North Carolina—systematically review the evidence of the effectiveness of a wide range of clinical preventive services, including screening, counseling, immunizations, and chemoprevention, in the primary care setting. -

Effects of Diethylstilbestrol, Norethindrone, and Mestranol on Selected Microbes
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1974 Effects of Diethylstilbestrol, Norethindrone, and Mestranol on Selected Microbes John N. Haan Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Physiology Commons Recommended Citation Haan, John N., "Effects of Diethylstilbestrol, Norethindrone, and Mestranol on Selected Microbes" (1974). Master's Theses. 2756. https://ecommons.luc.edu/luc_theses/2756 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1974 John N. Haan EFFECTS OF DIETHYLSTILBESTROL, NORETHINDRONE, AND MESTRANOL ON SELECTED MICROBES by John N. Haan A Thesis Submitted to the Faculty of the Graduate School of Loyola University of Chicago in Partial Fulfillment of the Requirements for the Degree of Master of Science November 1974 TABLE OF CONTENTS ,.' I. Introduction and Review of the Literature ................... 1 'II. Materials and Methods....................................... 7 III. Results· ..................................................... 13 A. Effects of Ethanol on the Microbes Studied .............. 13 1. Growth Studies ...................................... 13 B. Effects of Norethindrone