3B2 to Ps Tmp 1..11
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Commission Regulation (Ec)
17.3.2009EN Official Journal of the European Union L 71/15 COMMISSION REGULATION (EC) No 205/2009 of 16 March 2009 approving minor amendments to the specification for a name entered in the register of protected designations of origin and protected geographical indications (Riso Nano Vialone Veronese (PGI)) THE COMMISSION OF THE EUROPEAN COMMUNITIES, or maximum values instead of the average values provided to date. The third amendment concerns the physico-chemical parameters (amylase, gelatinisation, Having regard to the Treaty establishing the European consistency, viscosity). Community, (3) The Commission has examined the amendments in question and concluded that they are justified. Since Having regard to Council Regulation (EC) No 510/2006 of these are minor amendments within the meaning of 20 March 2006 on the protection of geographical indications Article 9 of Regulation (EC) No 510/2006, the and designations of origin for agricultural products and food Commission may approve them without using the stuffs (1), and in particular the second sentence of Article 9(2) procedure set out in Articles 5, 6 and 7 of that Regu thereof, lation, HAS ADOPTED THIS REGULATION: Whereas: Article 1 (1) In accordance with the first subparagraph of Article 9(1) The specification for the protected geographical indication ‘Riso and Article 17(2) of Council Regulation (EC) Nano Vialone Veronese’ is hereby amended in accordance with No 510/2006, the Commission has examined Italy’s Annex I to this Regulation. request for the approval of an amendment to the speci fication for the protected geographical indication ‘Riso Nano Vialone Veronese’, registered by Commission Regu Article 2 lation (EC) No 1107/96 (2), as amended by Commission A consolidated version of the summary containing the main Regulation (EC) No 1263/96 (3). -

AZIENDA U.L.S.S. N. 9 SCALIGERA Sede Legale: Via Valverde 42 – 37122 VERONA
AZIENDA U.L.S.S. n. 9 SCALIGERA Sede Legale: via Valverde 42 – 37122 VERONA DELIBERAZIONE DEL DIRETTORE GENERALE N. DEL Il Direttore Generale dell’Azienda U.L.S.S. n. 9 SCALIGERA, dott. Pietro Girardi, nominato con D.P.G.R.V. n. 28 del 26/02/2021, coadiuvato dai Direttori: - dott. Giuseppe Cenci Direttore Amministrativo - dott. Denise Signorelli Direttore Sanitario - dott. Raffaele Grottola Direttore dei Servizi Socio-Sanitari ha adottato in data odierna la presente deliberazione: OGGETTO PEDIATRIA DI LIBERA SCELTA – INDIVIDUAZIONE AMBITI TERRITORIALI CARENTI ANNO 2021 Note per la trasparenza: Con il presente provvedimento si individuano per l’anno 2021 gli ambiti territoriali carenti di Pediatria di Libera Scelta Il Direttore di UOC Direzione Amministrativa Territoriale Sentito il Direttore della Funzione Territoriale e il Responsabile UOS Medicina Convenzionata, Privati Accreditati e Controlli, Premesso che: - con nota n. 47579 di prot. del 2.2.2021 la Direzione Programmazione Sanitaria – LEA – Unità Organizzativa Cure Primarie e Strutture Socio-Sanitarie Territoriali – Area Sanità e Sociale della Regione del Veneto ha richiesto di inviare la rilevazione annuale di fini della pubblicazione relativa all’anno 2021 delle zone carenti ordinarie e straordinarie di pediatria di libera scelta; - con deliberazione n. 1128 del 31.12.2020 si era provveduto a rilevare le zone carenti straordinarie a seguito delle cessazioni dei pediatri di libera scelta a partire dall'1.9.2020 e fino al 31.12.2020, trasmesse ai competenti Uffici regionali con nota n. 9304 del 20.1.2021; - preso atto che i componenti del Comitato Aziendale, sentiti a mezzo posta elettronica in data 4.3.2021, considerata l’urgenza, hanno espresso parere favorevole in relazione all’individuazione degli ambiti territoriali carenti anno 2021 che, ivi compresi quelli già rilevati con la succitata deliberazione n. -

Linee E Orari Bus Extraurbani - Provincia Di Verona
Linee e orari bus extraurbani - Provincia di Verona Servizio invernale 2019/20 [email protected] 115 Verona - Romagnano - Cerro - Roverè - Velo ................................... 52 Linee da/per Verona 117 S.Francesco - Velo - Roverè - S.Rocco - Verona .............................. 53 101 S. Lucia - Pescantina - Verona ........................................................14 117 Verona - S.Rocco - Roverè - Velo - S.Francesco .............................. 53 101 Verona - Pescantina - S. Lucia ........................................................15 119 Magrano - Moruri - Castagnè - Verona .............................................54 102 Pescantina - Bussolengo - Verona .................................................. 16 119 Verona - Castagnè - Moruri - Magrano .............................................54 102 Verona - Bussolengo - Pescantina .................................................. 16 120 Pian di Castagnè/San Briccio - Ferrazze - Verona ............................ 55 90 Pescantina - Basson - Croce Bianca - Stazione P.N. - p.zza Bra - P. 120 Verona - Ferrazze - San Briccio/Pian di Castagnè ............................ 55 Vescovo ............................................................................................... 17 121 Giazza - Badia Calavena - Tregnago - Strà - Verona ......................... 56 90 P.Vescovo - p.zza Bra - Stazione P.N. - Croce Bianca - Basson - 121 Verona - Strà - Tregnago - Badia Calavena - Giazza ......................... 58 Pescantina ............................................................................................17 -

MARCON ING. PAOLO Via I° Maggio, 76/D – 37020 Volargne Di Dolcè (VR) Tel
MARCON ING. PAOLO Via I° Maggio, 76/d – 37020 Volargne di Dolcè (VR) Tel. 045 6861662 – Telefax. 045 6887665 Via delle Grazie, 3 - 38068 Rovereto (TN) • Tel. 0464 486506 [email protected] DATI PERSONALI: Nato a Rovereto (TN) il 7 agosto 1957 Residente a Rovereto (TN) - Via Rossini, 9 Iscr. Ordine degli Ingegneri di Verona e Provincia al n° 1590 Cod. Fisc.: MRC PLA 57M07H612M P.I.: 01237920234 STUDI REALIZZATI: Diploma di maturità scientifica conseguito presso il Liceo A. Rosmini di Rovereto (TN) nel 1976. Laureato presso l'Università degli studi di Padova nel 1983 in Ingegneria Civile Idraulica indirizzo Risorse idriche ed impatto ambientale. Corso di abilitazione ai sensi della legge 494/96 per gli obblighi di responsabile per la sicurezza in sede di progettazione ed esecuzione lavori conseguito presso la fondazione Edilscuola di Verona nell’anno 1997. Corso di aggiornamento ai sensi del D.lgs 81/08 per coordinatore per la progettazione e per l’esecuzione dei lavori presso la fondazione Edilscuola di Verona da ottobre 2011. ESPERIENZA PROFESSIONALE: Esercita la libera professione dal 1984 occupandosi di progettazione e direzione lavori nonché di coordinamento per la sicurezza. Dal 1984 è iscritto all'Ordine Ingegneri della Provincia di Verona al n° 1590 dove è membro nella Commissione LL.PP. Dal 1989 è socio dell'Associazione Idrotecnica Italiana. Iscritto nell’Elenco Regionale Consulenti e Prestatori di Servizi per la Regione Veneto al n° 492. Iscritto all’Albo dei Collaudatori Tecnici della Regione Veneto - categoria 5 n° 1627. Di seguito alcune delle opere progettate e dirette: Curriculum Professionale Servizi Tecnici - Ing. -

Publication of a Communication of Approval of a Standard Amendment to a Product Specification for a Name in the Wine Sector Refe
5.3.2020 EN Offi cial Jour nal of the European Union C 72/33 Publication of a communication of approval of a standard amendment to a product specification for a name in the wine sector referred to in Article 17(2) and (3) of Commission Delegated Regulation (EU) 2019/33 (2020/C 72/14) This communication is published in accordance with Article 17(5) of Commission Delegated Regulation (EU) 2019/33 (1). COMMUNICATING THE APPROVAL OF A STANDARD AMENDMENT ‘SOAVE’ Reference number: PDO-IT-A0472-AM05 Date of communication: 2 December 2019 DESCRIPTION OF AND REASONS FOR THE APPROVED AMENDMENT 1. Formal amendment For the ‘Classico’ type, the term ‘subarea’ has been replaced by the term ‘specification’. This is a formal amendment to take account of the exact wording provided in Italian law. This formal amendment concerns Article 1 of the Product Specification but it does not affect the Single Document. 2. Vine training systems The vine training systems have been extended to include GDC and all forms of trellises. The reason for this amendment is to adapt the Product Specification to the development of more modern and innovative vine training systems (particularly the various new kinds of trellises) and the need for agronomic methods to take account of climate change. This formal amendment concerns Article 4 of the Product Specification but it does not affect the Single Document. 3. Vine density per hectare As regards vine density per hectare (at least 3 300), the reference to the Decree of 7 May 1998 has been deleted. This is a formal amendment given that newly planted vines must have a minimum density of 3 300 plants per hectare. -

Servizio Di Continuità Assistenziale (Ex Guardia Medica)
COSA NON PUÒ FARE IL MEDICO SEDI DI CONTINUITÀ DI CONTINUITÀ ASSISTENZIALE? ASSISTENZIALE ULSS 22 Il medico di Continuità Assistenziale non può: Competenza territoriale SEDE Telefono per i comuni di: indirizzo • Prescrivere esami diagnostici, di laboratorio e strumentali, e visite specialistiche Bussolengo, Pastrengo, BUSSOLENGO Pescantina Via Ospedale, 28 045 6712606 SOMMACAMPAGNA Servizio di continuità • Prescrivere i farmaci con nota, salvo i casi previsti adeguatamente documen- Sommacampagna, Sona presso la Casa di Riposo, 045 8961902 Via Matteotti, 3 tati (esibendo diagnosi e/o piano terapeutico) CASTEL NUOVO DEL Bardolino, Castelnuovo del GARDA Garda, Lazise, Peschiera presso la Casa di Riposo, 045 6450712 assistenziale • Ripetere ricette mediche per tutti i farmaci assunti con continuità che non ri- Via Gianfilippi, 1 vestano il carattere della non differibilità Brenzone, Garda, Malcesine, MALCESINE San Zeno di Montagna, presso l’Ospedale, 045 6589342 Torri del Benaco Località Val di Sogno (ex guardia medica) Fumane, Marano di • Fare l’impegnativa per i ricoveri programmati che rimangono di esclusiva per- Valpolicella, Negrar, SAN PIETRO IN CARIANO tinenza del medico di famiglia San Pietro in Cariano, presso la Casa di Riposo, 045 7702558 Sant’Ambrogio di Valpolicella, Via Beethoven, 16 Sant’Anna d’Alfaedo Affi, Brentino Belluno, CAPRINO VERONESE • Rilasciare certificati INAIL Caprino Veronese, Cavaion, presso il Costermano, Dolcè, Ferrara di Centro Polifunzionale, 045 6207257 0 del 30 giugno 2009 rev. Monte Baldo, Rivoli Veronese Via Cappuccini, 1 Inoltre non è compito dei medici di Continuità Assistenziale trascrivere su ri- VILLAFRANCA Povegliano Veronese, presso l’Ospedale, Villafranca di Verona 045 6338111 cettari regionali i farmaci prescritti dai medici del Pronto Soccorso e dei reparti Via Ospedale, 2 ospedalieri. -

1570 10/08/2021
UOS Medicina Convenzionata, Privati Accreditati e controlli DETERMINAZIONE DIRIGENZIALE N. DEL OGGETTO: PEDIATRIA DI LIBERA SCELTA – ZONE CARENTI 2021 - INCARICO DEFINITIVO PER TRASFERIMENTO ALLA DOTT.SSA VOLTOLINA CLAUDIA PRESSO IL DISTRETTO N. 1 VERONA CITTA’ – AMBITO TERRITORIALE DIS_1_APLS_1 [COMUNI DI VERONA (CIRCOSCRIZIONI N. 6, 7 E 8), BOSCO CHIESANUOVA, CERRO VERONESE, ERBEZZO, GREZZANA, ROVERE’ VERONESE, SAN MARTINO BUON ALBERGO, LAVAGNO, VELO VERONESE)] CON VINCOLO DI APERTURA DELL’AMBULATORIO NEL COMUNE DI VERONA (CIRCOSCRIZIONE N. 6). Il Responsabile UOS Medicina Convenzionata e Privati Accreditati e Controlli, delegato, da ultimo, dal Direttore Generale dell’Azienda con deliberazione n. 4 del 01.03.2021, Premesso che: - con nota n. 20219 di prot. del 26.7.2021, acquisita al n. 131174 di prot., l’Azienda Zero, espletate le procedure di assegnazione degli ambiti territoriali in oggetto per conto della Regione del Veneto, ha comunicato il nominativo della Dott.ssa VOLTOLINA CLAUDIA quale avente diritto al conferimento del seguente incarico, per trasferimento, dal Distretto n. 2 dell’Est Veronese – ambito territoriale DIS_2_APLS_3 (con ambulatorio a Castel d’Azzano, attualmente in associazione semplice con i seguenti medici: dott.ssa ARCAMONE Loretta, dott.ssa DALLABERNARDINA Paola, dott.ssa SPOLETTINI Elena Nicoletta, dott. TODESCHINI Diego, dott.ssa TURCO Camilla), di Medico Pediatra di Libera Scelta convenzionato con l’Azienda U.L.S.S. n. 9 Scaligera presso il Distretto n. 1 Verona Città – ambito territoriale DIS_1_APLS_1 [Comuni di Verona (Circoscrizioni n. 6, 7 e 8), Bosco Chiesanuova, Cerro Veronese, Erbezzo, Grezzana, Roveré Veronese, San Martino Buon Albergo, Lavagno, Velo Veronese)] con vincolo di apertura dell’ambulatorio nel Comune di Verona (circoscrizione n. -

Linee E Orari Bus Extraurbani - Provincia Di Verona
Linee e orari bus extraurbani - Provincia di Verona Servizio Estivo 2016 [email protected] 141 Legnago - Roverchiara - Oppeano - Verona .....................................47 Linee da/per Verona 141 Verona - Oppeano - Roverchiara - Legnago .....................................47 102 Ceraino - Domegliara - S.Lucia - Pescantina - Bussolengo - Verona 143 Legnago - S.Pietro di Morubio - Bovolone - Verona ..........................48 ............................................................................................................. 12 144 Legnago - Cerea - Bovolone - Verona ..............................................49 102 Verona - Bussolengo - Pescantina - S.Lucia - Domegliara - Ceraino 144 Verona - Bovolone - Cerea - Legnago ..............................................50 ............................................................................................................. 12 146 Nogara - Isola d. Scala - Verona ......................................................51 Domegliara/Negrar - Verona ................................................................13 146 Verona - Isola d. Scala - Nogara ......................................................51 Verona - Negrar/Domegliara ................................................................15 148 Mantova - Castelbelforte - Trevenzuolo - Vigasio - Verona ................52 104 Fosse - S.Anna di Alfaedo - Negrar - Verona ....................................17 148 Verona - Vigasio - Trevenzuolo - Castelbelforte - Mantova ................52 104 Verona - Negrar - S.Anna -

Comunicato Stampa Per Il Giornale L'arena Da Parte Del Coordinamenti Dei Centri Servizi Della Provincia Di Verona
COMUNICATO STAMPA PER IL GIORNALE L'ARENA DA PARTE DEL COORDINAMENTI DEI CENTRI SERVIZI DELLA PROVINCIA DI VERONA. In questo momento di oggettiva difficoltà per tutti, i nostri Centri servizi stanno adottando ed hanno adottato fin da subito in maniera scrupolosa tutte le misure anti- contagio previste dalle linee guida dell’ISS e dalla Regione Veneto, andando in alcuni casi anche oltre il dovuto. I nostri residenti sono i soggetti più a rischio e per questo ogni struttura, a fronte di questa consapevolezza, pone la massima attenzione nell’applicare ogni misura atta ad impedire che l’infezione possa diffondersi all'interno dei nostri Enti. Dall’inizio dell’epidemia ad oggi abbiamo investito gran parte delle risorse disponibili nella prevenzione del contagio di ospiti e dipendenti, acquistando DPI, macchinari per la sanificazione ambientale e implementando piani formativi straordinari e procedure per il personale. Il personale sta affrontando con abnegazione e grande professionalità le difficoltà lavorative quotidiane che vedono al loro interno anche quelle generate dall'attuale emergenza epidemiologica in atto, sostenendo in ogni modo i nostri ospiti e a distanza i loro familiari. Le telefonate e le videochiamate sono in questo momento l’unico collegamento tra i familiari e gli ospiti e rappresentano un nuovo “modello” di comunicazione di questi tempi. Fatta questa doverosa premessa siamo a comunicare che i dati pubblicati oggi 07 aprile 2020 sul quotidiano l'Arena, riportanti il nome degli Enti con la presenza di casi di positività al -

Scuole Isola Della Scala (178.63
ATV - Azienda Trasporti Verona - Orario servizi speciali scuole 2021/2022 SCUOLE ISOLA DELLA SCALA LINEE EXTRAURBANE LINEA 146 LINEA 146 LINEA 158 LINEA 345 LINEA 347 LINEA 356 Nogara Stazione fs marc C3 Villafranca via Bixio Legnago Aut. Nogara Bovolone scuole Pellegrina via Della Valleverde Povegliano Aselogna Villimpenta Bovolone via Umberto Isola della Scala H via Tombetta Isolalta Cerea Bonferraro Crosare di Bovolone Isola della Scala CadiDavid Vigasio Casaleone Castel d'Ario Salizzole Marchesino Isola della Scala Sanguinetto Sorga Tarmassia Ist.Agr.Bovolino Engazzà Ponte Possero Casalbergo Buttapietra Concamarise Erbè Isola della Scala Pisona Salizzole Olmo Caselle di Is.d.Scala Tarmassia Isola della Scala Isola della Scala Isola della Scala CONSULTA GLI ORARI DELLE LINEE SU WWW.ATV.VERONA.IT O SCARICA L'APP INFOBUS VERONA ATV - Azienda Trasporti Verona - Orario servizi speciali scuole 2021/2022 SCUOLE ISOLA DELLA SCALA SPECIALI SCUOLE ISOLA DELLA SCALA entrata ORE 08:00 SC310 ISOLA DELLA SCALA SC310 ISOLA DELLA SCALA SC320 IST BOVOLINO SC310 ISOLA DELLA SCALA Raldon Campagnola 06:20 Nogarole Rocca 06:35 Villafranca via Bixio 06:48 Trenvenzuolo 06:59 Villafontana 06:27 Pradelle 06:40 Villafranca v. Mes. 06:51 Vigasio 07:08 Bovolone 06:30 Bagnolo 06:42 Povegliano 06:56 Olmo 07:18 Bovolone via Umberto 06:35 Roncolevà 06:50 Madonna d Uva Secca 07:00 Isola della Scala Sc 07:21 Fagnano 06:55 Azzano 07:04 Isola della Scala H 07:23 prosegue con linea Trenvenzuolo 06:59 via Marconi 07:06 Isola della Scala 07:25 356 Vigasio 07:08 Forette 07:10 Bovolone via Umberto 06:35 Olmo 07:18 Vigasio 07:15 Isola della Scala 06:55 Isola della Scala Sc 07:21 Olmo 07:25 Isola della Scala H 07:23 Isola della Scala Sc 07:28 Isola della Scala 07:25 Isola della Scala 07:31 SPECIALI SCUOLE ISOLA DELLA SCALA uscita ORE 13:00 SC310 MADONNA D UVA S. -
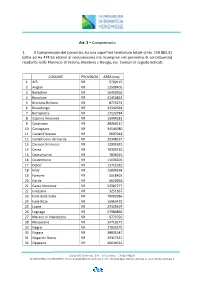
Comprensorio Consortile – Comuni E Superfici
Art. 3 – Comprensorio 1. Il Comprensorio del Consorzio, ha una superficie territoriale totale di Ha. 159.882,31 (oltre ad Ha 474,56 esterni al comprensorio ma ricompresi nel perimetro di contribuenza) ricadente nelle Provincie di Verona, Mantova e Rovigo, nei Comuni di seguito indicati: COMUNE PROVINCIA AREA (mq) 1 Affi VR 9799115 2 Angiari VR 12508405 3 Bardolino VR 16450266 4 Bovolone VR 41416891 5 Brentino Belluno VR 8773273 6 Bussolengo VR 24342564 7 Buttapietra VR 17132784 8 Caprino Veronese VR 13994181 9 Casaleone VR 38260117 10 Castagnaro VR 34544780 11 Castel d'Azzano VR 9697044 12 Castelnuovo del Garda VR 29348537 13 Cavaion Veronese VR 12902621 14 Cerea VR 70309710 15 Concamarise VR 7836525 16 Costermano VR 11036205 17 Dolce' VR 12711910 18 Erbe' VR 15804328 19 Fumane VR 5518402 20 Garda VR 6620294 21 Gazzo Veronese VR 53387277 22 Grezzana VR 3251367 23 Isola della Scala VR 70082086 24 Isola Rizza VR 16963410 25 Lazise VR 27325497 26 Legnago VR 57980860 27 Marano di Valpolicella VR 9773750 28 Mozzecane VR 24716373 29 Negrar VR 27826370 30 Nogara VR 38831547 31 Nogarole Rocca VR 29317657 32 Oppeano VR 46634522 Strada della Genovesa, 31/e – 37135 Verona – CF 93216480231 Tel. 0458569500 - Fax 0458569555 - Email [email protected] - Pec [email protected] – www.bonificaveronese.it 33 Palu' VR 13534474 34 Pastrengo VR 8972145 35 Pescantina VR 19711415 36 Peschiera del Garda VR 15309252 37 Povegliano Veronese VR 18652137 38 Rivoli Veronese VR 17264162 39 Ronco all'Adige VR 39510641 40 Roverchiara VR 18716636 -

Dati Del Segretario Comunale E Dipendenti Anno 2009
Comune di Nogara Provincia Verona Via Falcone-Borsellino, 1 37054 P. IVA 00660550237 0442513311 – Fax 0442/88333 _______________ E-mail: [email protected] OPERAZIONE TRASPARENZA ai sensi dell’art. 21, comma 1, L. 69 del 18.06.2009 IL NUMERO DEI DIPENDENTI COMUNALI QUALIFICA-PROFILO UOMINI DONNE TOTALE Tempo Part-time Tempo Part-time Tempo Part-time intero intero intero Segretario Comunale 0 1 0 0 0 1 Dirigenti 0 0 0 0 0 0 Istruttore Direttivo (Cat.D) 5 2 2 0 7 2 Assistente Sociale (Cat.D) 0 0 1 0 1 0 Istruttore Ammin.vo (Cat.C) 3 0 6 0 9 0 Educatrice Asilo Nido (Cat.C) 0 0 4 1 4 1 Assistente Tecnico (Cat.C) 1 0 0 0 1 0 Agente Polizia Locale (Cat.C) 0 0 3 0 3 0 Collaboratore (Cat.B/3) 5 0 5 0 8 0 Esecutore (Cat.B) 0 0 2 0 2 0 Ausiliaria (Cat.A) 0 0 1 0 1 0 TOTALE 14 3 24 1 38 4 LA DISTRIBUZIONE DEI DIPENDENTI COMUNALI NEGLI UFFICI 1° SETTORE AMMINISTRATIVO CONTABILE SERVIZI GENERAL I E TOTALE ISTITUZIONALI DIPENDENTI Segreteria – Organi Istituzionali - Personale – Cultura – Sport – 5 Associazionismo – Servizi Cimiteriali Polizia Amministrativa – Attività Produttive 1 Contabilità – Bilancio – Controllo di Gestione 2 Gestione Tributi 1 Servizi Polizia Locale 4 2° SETTORE DEMOGRAFICO - INFORMATICO TOTALE DIPENDENTI Servizi Demografici – Informatici 4 Servizio Protocollo – Ufficio Relazioni con il Pubblico 2 3° SETTORE LAVORI PUBBLICI – AMBIENTE – MANUTENZION E PATRIMONIO TOTALE DIPENDENTI Lavori Pubblici Ambiente Patrimonio 7 4° SETTORE SERVIZI SOCIALI - ISTRUZIONE TOTALE DIPENDENTI Servizi Sociali 4 Istruzione 8 5° SETTORE EDILIZIA