The Egretta Garzetta Complex in East Africa: a Case for One, Two Or Three Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Birds of the British Indian Ocean Territory, Chagos Archipelago, Central Indian Ocean Peter Carr
CARR: Birds of Chagos 57 Birds of the British Indian Ocean Territory, Chagos Archipelago, central Indian Ocean Peter Carr Carr, P., 2015. Birds of the British Indian Ocean Territory, Chagos Archipelago, central Indian Ocean. Indian BIRDS 10 (3&4): 57–70. Peter Carr, 80 Links Way, Beckenham, Kent, England, UK, BR3 3DQ. E-mail: [email protected]. Manuscript received on 02 June 2015. Introduction from three directions, the east, north and west and seabird The Chagos Archipelago lies at the end of the Chagos-Laccadive migrants from four, the north and south and dispersing east and Ridge and is some 500km due South of the Maldives archipelago. west along the equatorial counter current systems.” Observations It is the final termini for migrating organisms heading South in post-1971 have proven that Bourne’s words were prophetic; the central Indian Ocean. It is made up of five islanded atolls landbird and seabird vagrants and migrants are an exciting aspect centred upon the Great Chagos Bank, the largest atoll structure of birding in the Chagos. The vast majority of migratory species in the world. The climate is tropical oceanic, hot and humid yet are of northern hemisphere origin (though there is evidence moderated by trade winds. Mean monthly temperatures vary that a limited number of vagrants are from the east and west) from a maximum of 30.75°C in March to a minimum of 28.03°C and are generally present in the archipelago from September in August. The northern atolls of the archipelago are the wettest through to March. As more ornithological research is conducted in the Indian Ocean (Stoddart & Taylor 1971). -

UNEP/CBD/RW/EBSA/SIO/1/4 26 June 2013
CBD Distr. GENERAL UNEP/CBD/RW/EBSA/SIO/1/4 26 June 2013 ORIGINAL: ENGLISH SOUTHERN INDIAN OCEAN REGIONAL WORKSHOP TO FACILITATE THE DESCRIPTION OF ECOLOGICALLY OR BIOLOGICALLY SIGNIFICANT MARINE AREAS Flic en Flac, Mauritius, 31 July to 3 August 2012 REPORT OF THE SOUTHERN INDIAN OCEAN REGIONAL WORKSHOP TO FACILITATE THE DESCRIPTION OF ECOLOGICALLY OR BIOLOGICALLY SIGNIFICANT MARINE AREAS1 INTRODUCTION 1. In paragraph 36 of decision X/29, the Conference of the Parties to the Convention on Biological Diversity (COP 10) requested the Executive Secretary to work with Parties and other Governments as well as competent organizations and regional initiatives, such as the Food and Agriculture Organization of the United Nations (FAO), regional seas conventions and action plans, and, where appropriate, regional fisheries management organizations (RFMOs), with regard to fisheries management, to organize, including the setting of terms of reference, a series of regional workshops, with a primary objective to facilitate the description of ecologically or biologically significant marine areas (EBSAs) through the application of scientific criteria in annex I of decision IX/20, and other relevant compatible and complementary nationally and intergovernmentally agreed scientific criteria, as well as the scientific guidance on the identification of marine areas beyond national jurisdiction, which meet the scientific criteria in annex I to decision IX/20. 2. In the same decision (paragraph 41), the Conference of the Parties requested that the Executive Secretary make available the scientific and technical data and information and results collated through the workshops referred to above to participating Parties, other Governments, intergovernmental agencies and the Subsidiary Body on Scientific, Technical and Technological Advice (SBSTTA) for their use according to their competencies. -

"Official Gazette of RM", No. 28/04 and 37/07), the Government of the Republic of Montenegro, at Its Meeting Held on ______2007, Enacted This
In accordance with Article 6 paragraph 3 of the FT Law ("Official Gazette of RM", No. 28/04 and 37/07), the Government of the Republic of Montenegro, at its meeting held on ____________ 2007, enacted this DECISION ON CONTROL LIST FOR EXPORT, IMPORT AND TRANSIT OF GOODS Article 1 The goods that are being exported, imported and goods in transit procedure, shall be classified into the forms of export, import and transit, specifically: free export, import and transit and export, import and transit based on a license. The goods referred to in paragraph 1 of this Article were identified in the Control List for Export, Import and Transit of Goods that has been printed together with this Decision and constitutes an integral part hereof (Exhibit 1). Article 2 In the Control List, the goods for which export, import and transit is based on a license, were designated by the abbreviation: “D”, and automatic license were designated by abbreviation “AD”. The goods for which export, import and transit is based on a license designated by the abbreviation “D” and specific number, license is issued by following state authorities: - D1: the goods for which export, import and transit is based on a license issued by the state authority competent for protection of human health - D2: the goods for which export, import and transit is based on a license issued by the state authority competent for animal and plant health protection, if goods are imported, exported or in transit for veterinary or phyto-sanitary purposes - D3: the goods for which export, import and transit is based on a license issued by the state authority competent for environment protection - D4: the goods for which export, import and transit is based on a license issued by the state authority competent for culture. -

Report of the New York State Avian Records Committee for 2007
REPORT OF THE NEW YORK STATE AVIAN RECORDS COMMITTEE FOR 2007 The New York State Avian Records Committee (hereafter “NYSARC” or the “Committee”) reviewed 154 reports from 2007, involving 79 separate sightings, and an additional two reports from previous years. Reports were received from all over the state, with 31 of the 62 counties represented. The number of reports accompanied by photographs remains high. The Committee wishes to remind readers that reports submitted to eBird, listserves, local bird clubs, rare bird alerts (RBAs) and Kingbird Regional Editors are not necessarily forwarded to NYSARC, and doing so remains the responsibility of the observer. The growing use of the internet and mobile phones has had a very positive impact on the timely dissemination of rare bird sightings and has made it easier for birders to locate birds found by others. The Committee has always held that receipt of multiple independent reports provides a much fuller documentation of the sighting and can in some cases increase the likelihood of acceptance. We therefore urge ALL observers, not just the finder, to submit written reports and/or photographs. The names of the 83 contributors who submitted materials (written reports, photographs and sketches) are listed alongside accepted reports and again at the end of this document. Where possible, the name(s) of the original finder(s) is (are) included in the narratives. Production of this Annual Report is a team effort involving a large number of people. In addition to the contributors mentioned above, several Kingbird Regional Editors have made valued efforts in cajoling reluctant observers into preparing and submitting documentation. -

Effects of Nest Characteristics and Black Rat Rattus Rattus Predation on Daily Survival Rates of Great Egret Ardea Alba Nest
Effects of nest characteristics and black rat Rattus rattus predation on daily survival rates of great egret Ardea alba nests in mangrove forest in the Hara Biosphere Reserve, the Persian Gulf Authors: Elnaz Neinavaz, Ahmad Barati, Jessi L. Brown, Farzaneh Etezadifar, and Besat Emami Source: Wildlife Biology, 19(3) : 240-247 Published By: Nordic Board for Wildlife Research URL: https://doi.org/10.2981/12-087 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Wildlife-Biology on 04 Apr 2019 Terms of Use: https://bioone.org/terms-of-use Wildl. Biol. 19: 240-247 (2013) Original article DOI: 10.2981/12-087 Ó Wildlife Biology, NKV www.wildlifebiology.com Effects of nest characteristics and black rat Rattus rattus predation on daily survival rates of great egret Ardea alba nests in mangrove forest in the Hara Biosphere Reserve, the Persian Gulf Elnaz Neinavaz, Ahmad Barati, Jessi L. -
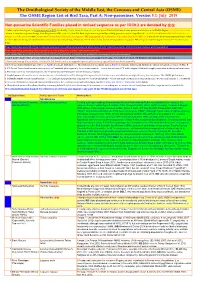
ORL 5.1 Non-Passerines Final Draft01a.Xlsx
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part A: Non-passerines. Version 5.1: July 2019 Non-passerine Scientific Families placed in revised sequence as per IOC9.2 are denoted by ֍֍ A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. English names shaded thus are taxa on BirdLife Tracking Database, http://seabirdtracking.org/mapper/index.php. Nos tracked are small. NB BirdLife still lump many seabird taxa. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. -

The Status of Coastal Birds on Uzi Island
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Spring 2014 The tS atus of Coastal Birds on Uzi Island: A Coastal Survey of Birds and Their nI teractions with Fishermen and Seaweed Farmers Anna Curtis-Heald SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Environmental Indicators and Impact Assessment Commons, and the Natural Resources and Conservation Commons Recommended Citation Curtis-Heald, Anna, "The tS atus of Coastal Birds on Uzi Island: A Coastal Survey of Birds and Their nI teractions with Fishermen and Seaweed Farmers" (2014). Independent Study Project (ISP) Collection. 1788. https://digitalcollections.sit.edu/isp_collection/1788 This Unpublished Paper is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. The Status of Coastal Birds on Uzi Island A coastal survey of birds and their interactions with fishermen and seaweed farmers Anna Curtis-Heald Connecticut College Advisors: Alawi Hija & Aliy Abdurahim Aliy SIT Zanzibar: Coastal Ecology and Natural Resource Management Academic Director: Nat Quansah Spring 2014 2 Table of Contents Acknowledgements…………………………………………3 Abstract……………………………………………………..4 Introduction…………………………………………………5 Study Area…………………………………………....……. .8 Methodology…………………………………………….....12 Results……………………………………………………...15 Discussion………………………………………………….25 Conclusion………………………………………………....33 Recommendations………………………………………….34 References………………………………………………….36 Appendixes…………………………………………………39 3 Acknowledgements I would like to thank Dr. Nat for leading us through this experience and for his words of wisdom. Thank you to Said for guiding me, making us feel at home, and always smiling. -

Waterbird Count Results in the Rift Valley, Nairobi and Central, Kenya for July 2018
The NATIONAL MUSEUMS of KENYA Waterbird Count Results in the Rift Valley, Nairobi and Central, Kenya for July 2018 Oliver Nasirwa CENTRE FOR BIODIVERSITY RESEARCH REPORTS: ORNITHOLOGY NO. 84, FEBRUARY 2019 Supported by: 1 Waterbird Count Results in the Rift Valley, Nairobi and Central, Kenya for July 2018: NMK Ornithology Reports No. 84, Feb. 2019 Waterbird Count Results in the Rift Valley, Nairobi and Central, Kenya for July 2018 Oliver Nasirwa National Museums of Kenya, PO Box 40658-00100, Nairobi, Kenya, [email protected]; NMK Centre for Biodiversity Research Reports: Ornithology No. 84, February 2019 Summary The July 2018 waterbird counts were carried out in 15 sites in the Rift Valley, Nairobi and Central, Kenya regions. Water levels were high in most sites during the counts particularly at lakes Baringo, Bogoria, Magadi and Ol’ Bolossat. A total of 808,862 individual waterbirds of 81 species were recorded across all the 15 sites. Lake Magadi had the highest number of individuals with 449,938 of 37 species followed by Lake Bogoria with 343,266 of 32 species and Lake Baringo with 5,702 of 44 species. The highest number of waterbird species was recorded at Lake Ol’ Bolossat with 50 species, followed by Lake Baringo with 44 species, and Lake Magadi and Dandora Sewerage Treatment Works with 37 species each. Across all the 15 sites, Lesser Flamingo Phoeniconaias minor was the most abundant species, dominating by 96.7% (781,921) of the total number of individuals counted followed by Greater Flamingo Phoenicopterus ruber with 1% (7,978) and Cattle Egret Bubulcus ibis with 0.2% (1,690). -

Species Present in the Barachois
2017 Species Present in the Barachois Antoine Rivière Internship of “the Barachois Project” EPCO 7/25/2017 Table of Contents Chapter 1: Sedentary Birds............................................................................................................................ 3 Scientific Name: Zosterops mauritianus ....................................................................................................... 4 Scientific Name: Foudia rubra (rare in the barchois) .................................................................................... 5 Scientific Name: Butorides striatus ............................................................................................................... 6 Scientific Name: Foudia Madagascariensis................................................................................................... 7 Scientific Name: Nesoenas picturata ............................................................................................................ 8 Scientific Name: Acridotheres tristis ............................................................................................................. 9 Scientific Name: Pycnonotus jocosus .......................................................................................................... 10 Scientific Name: Estrilda astrild .................................................................................................................. 11 Scientific Name: Ploceus cucullatus ........................................................................................................... -

Checklist of Amphibians, Reptiles, Birds and Mammals of New York
CHECKLIST OF AMPHIBIANS, REPTILES, BIRDS AND MAMMALS OF NEW YORK STATE Including Their Legal Status Eastern Milk Snake Moose Blue-spotted Salamander Common Loon New York State Artwork by Jean Gawalt Department of Environmental Conservation Division of Fish and Wildlife Page 1 of 30 February 2019 New York State Department of Environmental Conservation Division of Fish and Wildlife Wildlife Diversity Group 625 Broadway Albany, New York 12233-4754 This web version is based upon an original hard copy version of Checklist of the Amphibians, Reptiles, Birds and Mammals of New York, Including Their Protective Status which was first published in 1985 and revised and reprinted in 1987. This version has had substantial revision in content and form. First printing - 1985 Second printing (rev.) - 1987 Third revision - 2001 Fourth revision - 2003 Fifth revision - 2005 Sixth revision - December 2005 Seventh revision - November 2006 Eighth revision - September 2007 Ninth revision - April 2010 Tenth revision – February 2019 Page 2 of 30 Introduction The following list of amphibians (34 species), reptiles (38), birds (474) and mammals (93) indicates those vertebrate species believed to be part of the fauna of New York and the present legal status of these species in New York State. Common and scientific nomenclature is as according to: Crother (2008) for amphibians and reptiles; the American Ornithologists' Union (1983 and 2009) for birds; and Wilson and Reeder (2005) for mammals. Expected occurrence in New York State is based on: Conant and Collins (1991) for amphibians and reptiles; Levine (1998) and the New York State Ornithological Association (2009) for birds; and New York State Museum records for terrestrial mammals. -

SURVEY of LOZA BAY, NORTH WEST of MADAGASCAR by FELIX Razafindrajao, Durrell Wildlife Conservation Trust Programme Madagascar August 2015
WATERBIRD SURVEY OF LOZA BAY, NORTH WEST OF MADAGASCAR By FELIX Razafindrajao, Durrell Wildlife Conservation Trust Programme Madagascar August 2015 SUMMARY Through the ABC small grants scheme, three surveys of waterbirds were carried out in Loza Bay, north-west Madagascar in 2014 and 2015 (24 August to 02 September 2014 & 01-07 December 2014 and 10-15 February 2015). This was the first time that this important area of mangrove and bay has been surveyed for waterbirds. In total 37 waterbird species were recorded, belonging to 10 families. Four species are threatened with extinction; Madagascar fish-eagle Haliaeetus vociferoides (CR), Humblot’s heron Ardea humbloti (EN), Madagascar sacred ibis Threskiornis bernieri (EN), and Madagascar teal Anas bernieri (EN). Significant numbers of greater and lesser Flamingo Phoenicopterus ruber and Phoeniconaias minor were recorded during the surveys. In addition, 30 forest bird species were inventoried; all of them were endemic or regionally endemic species. Interviews were also conducted with local people, to collect information on their use of natural resources. Results from the interviews showed that local people are predominantly fishermen and there is significant pressure on natural resources through unsustainable use and disturbance. INTRODUCTION Loza Bay in the North West of Madagascar, district of Analalava and Antsohihy, is rich in biodiversity and plays an important role in the economy of the Sofia region. Three major rivers flow into the Loza Bay: the Tsinjomorona, the Maevarano and the Anjingo which feed into several wetland habitats for waterbirds. The bay is also important for transportation for local people as it links the Antsohihy and Analalava districts. -

Nakhiloo Coastal Waters IMMA
Nakhiloo Coastal Waters IMMA Summary (continued) in the area. Opportunistic sightings of both species further indicate their regular presence in the Nakhiloo National Marine Park. Both of these species are threatened by trawl fisheries, gillnet fisheries, and coastal development activities occurring in the IMMA. Description Area Size 401 km2 The Persian Gulf constitutes an almost closed body of shallow water with an average depth of 35m (Sheppard et al., 1992). These waters are subject to Qualifying Species and Criteria salinity fluctuations from 42 to 70 ppt and summer sea surface temperature ranges from 16°C to over Indian Ocean humpback dolphin – Sousa plumbea 36°C (Preen, 2004). A low tidal displacement in this Criteria A, B1 Gulf allows little discharge of its waters into the Indian Ocean and thus little opportunity to flush out Indo-Pacific finless porpoise – Neophocaena pollutants. The Nakhiloo marine national park is an phocaenoides Important Area for breeding marine bird species such Criteria A, C1 as terns (Sterna bengalensis, Sterna bergii, Onychoprion anaethetus), crab plover (Dromas ardeola), and the western reef heron (Egretta gularis). Marine Mammal Diversity (D2) Moreover, these waters are an important breeding Neophocaena phocaenoides, Sousa plumbea, area for hawksbill turtles (Eretmochelys imbricata) Balaenoptera edeni, Stenella longirostris, and foraging area for green sea turtles (Chelonia Delphinus delphis tropicalis mydas). Criterion A: Species or Population Summary Vulnerability This IMMA, located at the mouth of the Mond River The IMMA supports the year-round presence of Delta in the Bushehr province in southern Iran, Indian Ocean humpback dolphins (Sousa plumbea) encompasses the Dayer-Nakhiloo National Marine (Fig.