Final Report 2005-483-033 V1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Tale of Two Herichthys
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 209(1): 1-18 LIVE COLOR PATTERNS DIAGNOSE SPECIES: A TALE OF TWO HERICHTHYS By RONALD G. OLDFIELD1,2, ABHINAV KAKUTURU1, 2 3 4 WILLIAM I. LUTTERSCHMIDT , O. TOM LORENZ , ADAM E. COHEN , AND DEAN A. HENDRICKSON4 Department of Ecology and Evolutionary Biology and Museum of Zoology University of Michigan Ann Arbor, Michigan 48109–1079, USA Ann Arbor, April 27, 2021 ISSN 0076-8406 JOHN LUNDBERG1, EDITOR GERALD SMITH2, EDITOR MACKENZIE SCHONDELMAYER2, COMPOSITOR 1Department of Ichthyology, The Academy of Natural Sciences of Drexel University, Philadelphia, PA 19103 2Museum of Zoology, University of Michigan, Ann Arbor, MI 48197 LIVE COLOR PATTERNS DIAGNOSE SPECIES: A TALE OF TWO HERICHTHYS By RONALD G. OLDFIELD1,2, ABHINAV KAKUTURU1, WILLIAM I. LUTTERSCHMIDT2, O. TOM LORENZ3, ADAM E. COHEN4, AND DEAN A. HENDRICKSON4 ABSTRACT The Rio Grande Cichlid, Herichthys cyanoguttatus, is native to the drainages of the Gulf Coast of northern Mexico and southern Texas and has been introduced at several sites in the US. Previous observations have suggested that non-native populations in Louisiana that are currently recognized as H. cyanoguttatus resemble another species, the Lowland Cichlid, H. carpintis. Traditional morphological and genetic techniques have been insufficient to differentiate these species, but H. carpintis has been reported to differ fromH. cyanoguttatus in color pattern, so we turned to novel electronic photo archives to determine the identity of the species introduced in Louisiana. First, we used the public databases Nonindigenous Aquatic Species Database and Fishes of Texas to infer the historical distributions of these species in the US. -

Freshwater Ornamental Fish Commonly Cultured in Florida 1 Jeffrey E
Circular 54 Freshwater Ornamental Fish Commonly Cultured in Florida 1 Jeffrey E. Hill and Roy P.E. Yanong2 Introduction Unlike many traditional agriculture industries in Florida which may raise one or only a few different species, tropical Freshwater tropical ornamental fish culture is the largest fish farmers collectively culture hundreds of different component of aquaculture in the State of Florida and ac- species and varieties of fishes from numerous families and counts for approximately 95% of all ornamentals produced several geographic regions. There is much variation within in the US. There are about 200 Florida producers who and among fish groups with regard to acceptable water collectively raise over 800 varieties of freshwater fishes. In quality parameters, feeding and nutrition, and mode of 2003 alone, farm-gate value of Florida-raised tropical fish reproduction. Some farms specialize in one or a few fish was about US$47.2 million. Given the additional economic groups, while other farms produce a wide spectrum of effects of tropical fish trade such as support industries, aquatic livestock. wholesalers, retail pet stores, and aquarium product manufacturing, the importance to Florida is tremendous. Fish can be grouped in a number of different ways. One major division in the industry which has practical signifi- Florida’s tropical ornamental aquaculture industry is cance is that between egg-laying species and live-bearing concentrated in Hillsborough, Polk, and Miami-Dade species. The culture practices for each division are different, counties with additional farms throughout the southern requiring specialized knowledge and equipment to succeed. half of the state. Historic factors, warm climate, the proxim- ity to airports and other infrastructural considerations This publication briefly reviews the more common groups (ready access to aquaculture equipment, supplies, feed, etc.) of freshwater tropical ornamental fishes cultured in Florida are the major reasons for this distribution. -

Pathological Manifestations of Francisella Orientalis in the Green Texas Cichlid (Herichthys Cyanoguttatus)
animals Article Pathological Manifestations of Francisella orientalis in the Green Texas Cichlid (Herichthys cyanoguttatus) Chia-Hsuan Chang 1,† , Sayuj Poudyal 2,† , Theeraporn Pulpipat 3, Pei-Chi Wang 1,4,5,* and Shih-Chu Chen 1,2,4,5,6,* 1 Department of Veterinary Medicine, College of Veterinary Medicine, National Pingtung University of Science and Technology, Pingtung 91201, Taiwan; [email protected] 2 International Degree Program of Ornamental Fish Technology and Aquatic Animal Health, International College, National Pingtung University of Science and Technology, Pingtung 91201, Taiwan; [email protected] 3 Department of Farm Resources and Production Medicine, Faculty of Veterinary Medicine, Kasetsart University Kamphaeng Saen Campus, Nakhon Pathom 73140, Thailand; [email protected] 4 Southern Taiwan Fish Diseases Research Center, College of Veterinary Medicine, National Pingtung University of Science and Technology, Pingtung 91201, Taiwan 5 Research Center for Fish Vaccine and Diseases, College of Veterinary Medicine, National Pingtung University of Science and Technology, Pingtung 91201, Taiwan 6 Research Center for Animal Biologics, National Pingtung University of Science and Technology, Pingtung 91201, Taiwan * Correspondence: [email protected] (P.-C.W.); [email protected] (S.-C.C.); Tel.: +886-8-7740569 (P.-C.W. & S.-C.C.) † These authors contributed equally. Simple Summary: The following study demonstrates the pathological manifestations of an emerg- Citation: Chang, C.-H.; Poudyal, S.; ing virulent bacterium, Francisella orientalis, in an ornamental cichlid fish, the green Texas cichlid Pulpipat, T.; Wang, P.-C.; Chen, S.-C. (Herichthys cyanoguttatus). This study was conducted to prove that Francisella orientalis can cause a dis- Pathological Manifestations of ease in the green Texas cichlid that is similar to natural infection. -

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

FRESHWATER Pricelist 08.10.20
SUPER LOT SPECIALS GO Aquatics LLC 228364.1 Crowntail Betta, Male +12 Lot Phone: 612-379-1315 Fax: 612-379-1365 [email protected]_______________ 267304.1 Oratipinnis Bicher +3 Lot Specials 08/10/2020 – 08/15/2020 494002.1 Nymph Goldfish, S +25 Lot Buy any GLO Tetra 25+ to receive lot pricing Price(Longfin not included) ------------ -------------------------------------- 120274 Gold Chinese Algae Eater We have a large amount of Used 20 gallon long tanks that are drilled on the bottom if 120293 Garra Rufa anyone wants. Priced to sell! They need to be 121203 Bushnose Pleco, MS Picked up unless it is something we are 160254.8 Black Widow Frontosa, M delivering locally. 161964 Mel. Auratus, M There is a 10 lot minimum on ALL items $0.70 162404 Ps. Acei, M and under, and $150.00 order minimum on all GO Aquatics deliveries, not counting frozen 163364 Ps. Socoloffi, M and animals. 167303 Jewel Cichlid, MS 170366 Electric Blue Acara, L 172803 Black Convict, MS 172853 Pink Convict, MS 180213 Green Severum(Peru) MS 190404 Giant Danio 228364 Crowntail Betta, Male 253606 Black Kuhli Loach 267304 Ornatipinnis Bicher 290803 Bala Shark, MS 341607 Buenos Aires Tetra 342306 Red & Blue Columbian Tetra 347304 Gold Pristella Tetra 349374 Painted White Skirt 492503 Calico Fantail, USA, MS 454504 Red Oranda, M 494002 Nymph Goldfish, S 572002 Moss Ball, S 602300 Feeder Live Bearer 616303 Frozen Rat, S 616304 Frozen Rat, M 714004 Pacman Frog 714104 Albino PacMan Frog SpeeDee Customers !1 We highly urge you to make sure your order is 104804 Mixed Angel, Veil, M sent to our office 24 hours before you would like it shipped. -
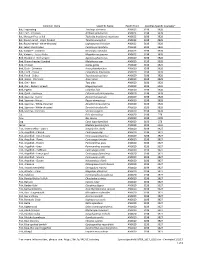
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -
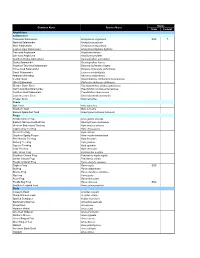
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Changes in the Fish Community of a Western Caribbean Estuary After the Expansion of an Artificial Channel to the Sea
water Article Changes in the Fish Community of a Western Caribbean Estuary after the Expansion of an Artificial Channel to the Sea Juan J. Schmitter-Soto * and Roberto L. Herrera-Pavón El Colegio de la Frontera Sur, Av. Centenario km 5.5, Chetumal 77014, Quintana Roo, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +52-983-835-0440 (ext. 4302) Received: 30 October 2019; Accepted: 2 December 2019; Published: 6 December 2019 Abstract: Increased connectivity between coastal lagoons and the sea is expected to entail a greater proportion of marine species in the former. Chetumal Bay, estuary of the Hondo river into the Caribbean, had a limited access to the sea until the opening of the Zaragoza Canal. We sought changes in the fish community from 1999–2001 (just after an expansion of the canal) to 2015–2018. The same fishing gear was used, in the same localities, during all seasons. Total fish abundance and mean local richness decreased, although total abundance increased in the polyhaline zone. Diversity was greater in the oligohaline zone in 1999–2001, and in the mesohaline zone in 2015–2018. Three guilds were absent in 2015–2018: Medium-sized herbivores, large piscivores, and medium-sized planktivores. Abundance of small benthivores decreased by decade; medium-sized piscivores and small planktivores became more abundant in 2015–2018 in the polyhaline zone. These changes may be due to the opening of the channel, but illegal fishing outside the bay may explain the decrease in juveniles of large piscivores, and erosion in the innermost part may be destroying important habitats. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -

Fishes of the Lemon Bay Estuary and a Comparison of Fish Community Structure to Nearby Estuaries Along Florida’S Gulf Coast
Biological Sciences Fishes of the Lemon Bay estuary and a comparison of fish community structure to nearby estuaries along Florida’s Gulf coast Charles F. Idelberger(1), Philip W. Stevens(2), and Eric Weather(2) (1)Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, Charlotte Harbor Field Laboratory, 585 Prineville Street, Port Charlotte, Florida 33954 (2)Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 100 Eighth Avenue Southeast, Saint Petersburg, Florida 33701 Abstract Lemon Bay is a narrow, shallow estuary in southwest Florida. Although its fish fauna has been studied intermittently since the 1880s, no detailed inventory has been available. We sampled fish and selected macroinvertebrates in the bay and lower portions of its tributaries from June 2009 through April 2010 using seines and trawls. One hundred three fish and six invertebrate taxa were collected. Pinfish Lagodon rhomboides, spot Leiostomus xanthurus, bay anchovy Anchoa mitchilli, mojarras Eucinostomus spp., silver perch Bairdiella chrysoura, and scaled sardine Harengula jaguana were among the most abundant species. To place our information into a broader ecological context, we compared the Lemon Bay fish assemblages with those of nearby estuaries. Multivariate analyses revealed that fish assemblages of Lemon and Sarasota bays differed from those of lower Charlotte Harbor and lower Tampa Bay at similarities of 68–75%, depending on collection gear. These differences were attributed to greater abundances of small-bodied fishes in Lemon and Sarasota bays than in the other much larger estuaries. Factors such as water circulation patterns, length of shoreline relative to area of open water, and proximity of Gulf passes to juvenile habitat may differ sufficiently between the small and large estuaries to affect fish assemblages. -

Texas Safari: the Fishing Guide to Texas by Gayne C. Young Copyright 2011 Gayne C
Texas Safari: The Fishing Guide to Texas By Gayne C. Young Copyright 2011 Gayne C. Young Smashwords Edition www.gaynecyoung.com Smashwords Edition, License Notes Thank you for downloading this free ebook. You are welcome to share it with your friends. This book may be reproduced, copied and distributed for non-commercial purposes, provided the book remains in its complete original form. If you enjoyed this book, please return to Smashwords.com to discover other works by this author. Thank you for your support. Contents Acknowledgements Introduction Texas Waters and Fishing Chapter 1: Notes on Means and Methods Sidebar: Fishing with Carrots Sidebar: It’s Stupid and Illegal Notes on Boats Chapter 2: Fresh Water Major Rivers Brazos Canadian Colorado Guadalupe Sidebar: Texas Water Safari Neches Nueces Sidebar: Mosquitoes Suck Pecos Sidebar: Hire a Guide Red Sidebar: The Caddo Lake Pearl Rush of 1909 Rio Grande Sabine San Antonio Sidebar: Woman Hollering Creek San Jacinto Trinity Sidebar: Remember Kids, Don’t Make Deals with Catfish Major Lakes Sidebar: Amistad, Then and Now Sidebar: Concho Pearl Sidebar: The Creature from Lake Worth Chapter 3: Freshwater Species Black Bass Largemouth Sidebar: Budweiser ShareLunker Program Smallmouth Spotted Sidebar: Calling all Fish Guadalupe True Bass White Yellow Striped Sidebar: Yeah, But it was a Mean Snake Hybrid Catfish & Bullheads Blue Sidebar: The Life and Times of Splash the Catfish Channel Flathead Sidebar: Freshwater Man-Eaters Black bullhead Yellow bullhead Crappie Black White Sidebar: Watching -
![Kyfishid[1].Pdf](https://docslib.b-cdn.net/cover/2624/kyfishid-1-pdf-1462624.webp)
Kyfishid[1].Pdf
Kentucky Fishes Kentucky Department of Fish and Wildlife Resources Kentucky Fish & Wildlife’s Mission To conserve, protect and enhance Kentucky’s fish and wildlife resources and provide outstanding opportunities for hunting, fishing, trapping, boating, shooting sports, wildlife viewing, and related activities. Federal Aid Project funded by your purchase of fishing equipment and motor boat fuels Kentucky Department of Fish & Wildlife Resources #1 Sportsman’s Lane, Frankfort, KY 40601 1-800-858-1549 • fw.ky.gov Kentucky Fish & Wildlife’s Mission Kentucky Fishes by Matthew R. Thomas Fisheries Program Coordinator 2011 (Third edition, 2021) Kentucky Department of Fish & Wildlife Resources Division of Fisheries Cover paintings by Rick Hill • Publication design by Adrienne Yancy Preface entucky is home to a total of 245 native fish species with an additional 24 that have been introduced either intentionally (i.e., for sport) or accidentally. Within Kthe United States, Kentucky’s native freshwater fish diversity is exceeded only by Alabama and Tennessee. This high diversity of native fishes corresponds to an abun- dance of water bodies and wide variety of aquatic habitats across the state – from swift upland streams to large sluggish rivers, oxbow lakes, and wetlands. Approximately 25 species are most frequently caught by anglers either for sport or food. Many of these species occur in streams and rivers statewide, while several are routinely stocked in public and private water bodies across the state, especially ponds and reservoirs. The largest proportion of Kentucky’s fish fauna (80%) includes darters, minnows, suckers, madtoms, smaller sunfishes, and other groups (e.g., lam- preys) that are rarely seen by most people.