Paycheck Protection Program Loans: Frequently Asked Questions (Faqs)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IHS Covid-19 Response 100 Day Review
INDIAN HEALTH SERVICE COVID-19 RESPONSE, 100 DAY REVIEW PLANNING SECTION Table of Contents Introduction ................................................................................................................................................. 2 Executive Summary .................................................................................................................................... 2 Summary of activities by aim ................................................................................................................. 3 Indian Health Service Response to COVID-19 ........................................................................................ 4 COVID-19 Funding ................................................................................................................................ 5 Aims and Strategic Objectives of the IHS Action Plan ....................................................................... 6 Aim 1: To Prevent the Spread of COVID-19 ....................................................................................... 7 Aim 2: To Detect Cases of COVID-19 ................................................................................................... 8 Aim 3: To Treat COVID-19 Cases and Sustain Regular Operations ............................................... 10 Aim 4: To Support the Indian Health System in the Recovery from COVID-19 ........................... 11 Aim 5: To Manage Resources ............................................................................................................. -

From the Factory to the Frontlines the Operation Warp Speed Strategy for Distributing a COVID-19 Vaccine
From the Factory to the Frontlines The Operation Warp Speed Strategy for Distributing a COVID-19 Vaccine What This Strategy Aims to Do This report to Congress details a strategy to achieve the principal purpose and objective of Operation Warp Speed (OWS): ensuring that every American who wants to receive a COVID-19 vaccine can receive one, by delivering safe and effective vaccine doses to the American people beginning January 2021. The leadership of OWS has committed to being transparent with Congress, the media, and the American people. OWS has provided regular briefings on topics of interest to Congress and the media and will continue to provide updates and announcements as OWS reaches new milestones. Congress has been a vital partner in the all-of-America response to the COVID-19 pandemic. With support provided through emergency supplemental and flexible discretionary funding, OWS has now made strong progress toward a safe and effective COVID-19 vaccine, with multiple candidates in Phase 3 clinical trials. Simultaneously, OWS and partners are developing a plan for delivering a safe and effective product to Americans as quickly and reliably as possible. Experts from the Department of Health and Human Services (HHS) are leading vaccine development, while experts from the Department of Defense (DoD) are partnering with the Centers for Disease Control and Prevention (CDC) and other parts of HHS to coordinate supply, production, and distribution of vaccines. Successful implementation of the national COVID-19 vaccination program requires precise coordination across federal, state, local, tribal, and territorial governments and among many public and private partners. -

COVID-19 UPDATE November 23, 2020 Global Total Cases – 59,025,871 Total Deaths – 1,393,889
COVID-19 UPDATE November 23, 2020 Global Total cases – 59,025,871 Total deaths – 1,393,889 United States Total cases – 12,369,978 Total deaths – 257,415 On Wednesday, the United States surpassed 250,000 coronavirus deaths. The total number of patients hospitalized with COVID-19 in the United States has reached new highs every day since November 11, when hospitalizations first surpassed the April peak. One week before the end of the month, the United States has already recorded its highest monthly case total in November, reporting more than 3,075,000 cases. The number of November cases could exceed four million, more than double the total in October. Trump Administration • The Centers for Disease Control and Prevention urged Americans against traveling for Thanksgiving and to limit gatherings to people in the same household as Covid-19 rages in the U.S. o “Celebrating virtually or with the people you live with is the safest choice this Thanksgiving,” the agency said on its website on Thursday. “Travel may increase your chance of getting and spreading COVID-19.” o For people considering traveling for the holiday, the agency offered a checklist of risks and standard advice such as wearing a face covering and staying 6 feet away from people “who don’t live with you.” People hosting Thanksgiving guests at home should observe precautions that could include having an outdoor meal with family and friends, have people bring their own food and drink, and opening windows if the gathering is indoors. • In a call last week, Centers for Disease Control and Prevention Director Robert Redfield and Health and Human Services Secretary Alex Azar encouraged governors to help build confidence in vaccines and stressed the importance of wearing masks. -

COVID-19: a Weekly Health Care Update from Washington April 13-17, 2020
COVID-19: A Weekly Health Care Update from Washington April 13-17, 2020 IN BRIEF What Happened This Week: Negotiators failed to reach consensus on a proposal to provide additional support for small businesses this week (hospital funding was one of the major sticking points, although negotiations are progressing). Meanwhile, at the White House, President Trump and members of the Coronavirus Task Force unveiled the details of a new phased approach to “reopen” the nation’s the economy yesterday and instructed state governors to take the lead. What to Expect in the Weeks and Months Ahead: Expect lawmakers to continue negotiating a path forward for additional small business support; expect lawmakers to continue working remotely on additional measures tied to the pandemic; and expect the Trump Administration to continue providing clarity and guidance on the distribution of funds and implementation of other major provisions in the first three COVID-19 bills. DEEP DIVE President Trump Unveils “Guidelines for Opening Up America Again”; Instructs State Governors to Take the Lead on a Phased Reopening of the Economy President Trump and members of the White House Coronavirus Task Force unveiled the details of a new phased “reopening” of the nation’s economy using a “deliberate, data-driven approach.” The Administration leaves much of the decision-making to the states but instructs them to open up gradually after benchmarks on new cases, testing, and hospital resources are met. As far as timing goes, Coronavirus Task Force Response Coordinator Dr. Deborah Birx said there is no set schedule for any of the guideline’s three phases. -

UMBC Alumnae Racing to Develop Coronavirus Vaccine
Newsletter SPRING 2020 To our UMBC/Meyerhoff families: We hope you and your families are all doing well during this strange and stressful time of Covid- 19. Although the world has changed quickly with so many things shut down and many of us sheltering at home, we hope this newsletter will represent a ray of sunshine during a dark and difficult time. Please enjoy this positive representation of our student and alumni community. MPA Board UMBC Alumnae Racing to Develop Coronavirus Vaccine Kizzmekia Corbett ’08, M16, biological sciences, says it feels like she’s “living in a constant adrenaline rush.” Maybe that’s because she and her team at the Vaccine Research Center at the National Insti- tute of Allergy and Infectious Diseases have been working around the clock for weeks. They’re racing to develop a vaccine for the coronavirus faster than it can race across the globe. “To be living in this moment where I have the opportunity to work on something that has imminent global importance…it’s just a surre- al moment for me,” Corbett says. Despite it feeling surreal, the advances Corbett and her team are making are very real, and they’re setting records. “We are making better progress than I could have ever hoped for,” she says. After three months of studies in test tubes and in animals, the vaccine her team developed is about to enter a phase I clinical trial, a crucial hur- dle on the way to FDA approval. Read the complete article about Kizzmekia and her team’s efforts to develop a Covid-19 vaccine in the latest UMBC magazine at https:// Kizzmekia Corbett, NIH magazine.umbc.edu/umbc-alumnae-racing-to-develop- coronavirus-vaccine/. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

Community Report March, 12 2021
COMMUNITY REPORT March 12, 2021 ONE YEAR LATER - A COMMUNITY ASSEMBLED On January 21, 2020, the United States reported its first case of the novel coronavirus that causes the disease COVID-19. By March 10, the first case in Michigan was reported and three days later, key community partners from health, government, first responders, education, social service, and business sectors in Grand Traverse County assembled as the Joint Operations Center (JOC). From March 13th until April 17th, the group would meet daily sharing information and resources. From briefings from the Health Department on the latest information surrounding the pandemic to coordinating efforts to provide the healthcare field with necessary PPE (personal protective equipment), to assuring that students that relied on school lunches for meals would not go hungry, the group cohesively worked together to navigate the social disruption the pandemic would bring to the world and our community. Over the past year, meetings slowly began to scale back to five days a week, then to three and now, just a single meeting on Fridays with sometimes over 100 community representatives listening in to the key contributors likely remains one of the most impactful mechanisms Grand Traverse County has had over the past year to put our community in the best position to face the pandemic head on. “I don't think we will ever know the degree of positive impact the establishment of the JOC had on our community. As partners and community leaders, the JOC members engaged the challenge of addressing the layers of issues that the pandemic has caused. -

March 12, 2019 / Rules and Regulations 8813
Federal Register / Vol. 84, No. 48 / Tuesday, March 12, 2019 / Rules and Regulations 8813 appropriate, disproportionate human the U.S. House of Representatives, and reference, Intergovernmental relations, health or environmental effects, using the Comptroller General of the United Particulate matter, Reporting and practicable and legally permissible States prior to publication of the rule in recordkeeping requirements. methods, under Executive Order 12898 the Federal Register. A major rule Dated: February 21, 2019. (59 FR 7629, February 16, 1994). cannot take effect until 60 days after it Cheryl L. Newton, In addition, the SIP is not approved is published in the Federal Register. to apply on any Indian reservation land This action is not a ‘‘major rule’’ as Acting Regional Administrator, Region 5. or in any other area where EPA or an defined by 5 U.S.C. 804(2). 40 CFR part 52 is amended as follows: Indian tribe has demonstrated that a Under section 307(b)(1) of the CAA, tribe has jurisdiction. In those areas of petitions for judicial review of this PART 52—APPROVAL AND Indian country, the rule does not have action must be filed in the United States PROMULGATION OF tribal implications and will not impose Court of Appeals for the appropriate IMPLEMENTATION PLANS substantial direct costs on tribal circuit by May 13, 2019. Filing a governments or preempt tribal law as petition for reconsideration by the ■ 1. The authority citation for part 52 specified by Executive Order 13175 (65 Administrator of this final rule does not continues to read as follows: FR 67249, November 9, 2000). -

Monday, April 5, 2021
Monday, April 5, 2021 WHERE WE ARE TODAY 3 April 5, 2021 OUR PROGRESS ON VACCINATIONS 4 # DC residents partially or fully vaccinated January 1 5,846 February 1 40,839 March 1 74,811 April 1 162,669 (Apr 2) Data source: DC Health, using received data through 4/2/2021. Data subject to change. April 5, 2021 5 We have more work to do to get all residents vaccinated. Make sure you’re signed up to get your COVID-19 shot. Go to: Call: vaccinate.dc.gov 1-855-363-0333 April 5, 2021 PRE-REGISTRATION SNAPSHOT 6 Approximately 190,621 people have pre-registered for a vaccination appointment and are awaiting an appointment. April 5, 2021 7 If you pre-registered for a vaccination appointment but already received your vaccine elsewhere you can email [email protected] to remove yourself from the pre-registration list. April 5, 2021 NEW AND UPCOMING CLINICS 8 This week, the vaccine clinic at the Convention Center will expand through a partnership with Safeway. Appointment invitations will be sent on April 6. On Friday, April 9, a new high-capacity vaccination site will open at Arena Stage. The site will be operated in partnership with DC Health and MedStar Health. Appointments will be made through vaccinate.dc.gov and the District’s call center. April 5, 2021 THE PATH FORWARD FIVE FACTORS DETERMINING AN ACTIVITY’S RE-OPEN POTENTIAL 10 1. MASKS - Can and is the activity completed with or without wearing a mask? Highest Risk Unmasked, Indoors, Long Time, Very Dense 2. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
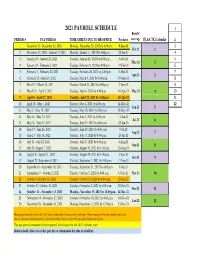
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -

COVID-19: Federal Efforts Accelerate Vaccine and Therapeutic
United States Government Accountability Office Report to Congressional Addressees November 2020 COVID-19 Federal Efforts Accelerate Vaccine and Therapeutic Development, but More Transparency Needed on Emergency Use Authorizations GAO-21-207 November 2020 COVID-19 Federal Efforts Accelerate Vaccine and Therapeutic Development, but More Transparency Needed on Highlights of GAO-21-207, a report to Emergency Use Authorizations congressional addressees Why GAO Did This Study What GAO Found The U.S. had about 10.3 million Through Operation Warp Speed—a partnership between the Department of cumulative reported cases of COVID- Health and Human Services (HHS) and the Department of Defense (DOD)—the 19 and about 224,000 reported deaths federal government is accelerating efforts to develop vaccines and therapeutics as of November 12, 2020. Given this for COVID-19. A typical vaccine development process can take approximately 10 catastrophic loss of life as well as the years or longer, but efforts under Operation Warp Speed seek to greatly pandemic’s effects on the U.S. accelerate this process by completing key steps simultaneously (see figure). As economy, effective and safe vaccines of October 15, 2020, Operation Warp Speed publicly announced financial and therapeutics are more important support for the development or manufacturing of six COVID-19 vaccine than ever. candidates totaling more than $10 billion in obligations. It has also announced The CARES Act includes a provision financial support for the development of therapeutics, such as a $450 million for GAO to report on its ongoing award to manufacture a monoclonal antibody treatment (a treatment that uses monitoring and oversight efforts related laboratory-made antibodies, which also may be able to serve as a prevention to the COVID-19 pandemic.