Aromaticity of Polycyclic Conjugated Hydrocarbons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Characterization of Heat Reformed Naphtha Cracking Bottom Oil Extracts
Carbon Vol. 9, No. 4 December 2008 pp. 289-293 Letters Characterization of Heat Reformed Naphtha Cracking Bottom Oil Extracts Jong Hyun Oh 1, Jae Young Lee1, Seok Hwan Kang2, Tai Hyung Rhee3 and Seung Kon Ryu1,♠ 1Departmentl of Chemical Engineering, Chungnam National University, Daejeon 305-764, Korea 2Uniplatek co., Ltd. Of “themoQ” 104-10 Munji-dong Yuseong, Daejeon 305-380, Korea 3Chemical Matrials Team, TECHNO SEMICHEM Co., Ltd., Kongju 314-240, Korea ♠email: [email protected] (Received November 13, 2008; Accepted December 12, 2008) Abstract Naphtha Cracking Bottom (NCB) oil was heat reformed at various reforming temperature and time, and the volatile extracts were characterized including yields, molecular weight distributions, and representative compounds. The yield of extract increased as the increase of reforming temperature (360 ~ 420oC) and time (1 ~ 4 hr). Molecular weight of the as-received NCB oil was under 200, and those of extracts were distributed in the range of 100-250, and far smaller than those of precursor pitches of 380-550. Naphtalene-based compounds were more than 70% in the as-received NCB oil, and most of them were isomers of compounds bonding functional groups, such as methyl (CH3-) and ethyl (C2H5-). When the as-received NCB oil was reformed at 360oC for 1 hr, the most prominent compound was 1,2-Butadien, 3-phenyl- (24.57%), while naphthalene became main component again as increasing the reforming temperature. Keywords : NCB oil, pitch, extract analysis, heat reforming 1. Introduction preparation of precursor pitch for pitch-based carbon fiber. The extracts were characterized to investigate the molecular 860 million barrel per year of petroleum oil was refined in weight distribution, representative compounds, yield, and Korea, 2004 [1]. -

Messengers from Outer Space
The complete absence of national laboratories for standardizing radiation measurements and the lack of physics departments in most radiotherapy centres in Latin America warrant the setting up of one or more regional dosimetry facilities, the functions of which would be primarily to calibrate dosimeters; to provide local technical assistance by means of trained staff; to check radiation equipment and dosimeters; to arrange a dose-intercomparison service; and to co-operate with local personnel dosimetry services. To be most effective, this activity should be in the charge of local personnel, with some initial assistance in the form of equipment and experts provided by the Agency. Although the recommendations were presented to the IAEA as the or ganization responsible for the setting up of the panel, the participants re commended that the co-operation of the World Health Organization and the Pan-American Health Organization should be invited. It was also suggested that the panel report be circulated to public health authorities in the countries represented. MESSENGERS FROM OUTER SPACE Although no evidence has yet been confirmed of living beings reaching the earth from space, it has been estimated that hundreds of tons of solid matter arrive every day in the form of meteorites or cosmic dust particles. Much is destroyed by heat in the atmosphere but fragments recovered can give valuable information about what has been happening in the universe for billions of years. Some of the results of worldwide research on meteorites were given at a symposium in Vienna during August. During six days of discussions a total of 73 scientific papers was present ed and a special meeting was held for the purpose of improving international co-operation in the research. -
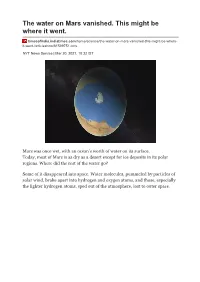
The Water on Mars Vanished. This Might Be Where It Went
The water on Mars vanished. This might be where it went. timesofindia.indiatimes.com/home/science/the-water-on-mars-vanished-this-might-be-where- it-went-/articleshow/81599751.cms NYT News Service | Mar 20, 2021, 10:32 IST Mars was once wet, with an ocean’s worth of water on its surface. Today, most of Mars is as dry as a desert except for ice deposits in its polar regions. Where did the rest of the water go? Some of it disappeared into space. Water molecules, pummeled by particles of solar wind, broke apart into hydrogen and oxygen atoms, and those, especially the lighter hydrogen atoms, sped out of the atmosphere, lost to outer space. A tall outcropping of rock, with layered deposits of sediments in the distance, marking a remnant of an ancient, long-vanished river delta in Jezero Crater, are pictured in this undated image taken by NASA's Mars rover Perseverance. (Reuters) But most of the water, a new study concludes, went down, sucked into the red planet’s rocks. And there it remains, trapped within minerals and salts. Indeed, as much as 99% of the water that once flowed on Mars could still be there, the researchers estimated in a paper published this week in the journal Science. Data from the past two decades of robotic missions to Mars, including NASA ’s Curiosity rover and the Mars Reconnaissance Orbiter, showed a wide distribution of what geologists call hydrated minerals. “It became very, very clear that it was common and not rare to find evidence of water alteration,” said Bethany Ehlmann, a professor of planetary science at the California Institute of Technology and one of the authors of the paper. -

Aromaticity Sem- Ii
AROMATICITY SEM- II In 1931, German chemist and physicist Sir Erich Hückel proposed a theory to help determine if a planar ring molecule would have aromatic properties .This is a very popular and useful rule to identify aromaticity in monocyclic conjugated compound. According to which a planar monocyclic conjugated system having ( 4n +2) delocalised (where, n = 0, 1, 2, .....) electrons are known as aromatic compound . For example: Benzene, Naphthalene, Furan, Pyrrole etc. Criteria for Aromaticity 1) The molecule is cyclic (a ring of atoms) 2) The molecule is planar (all atoms in the molecule lie in the same plane) 3) The molecule is fully conjugated (p orbitals at every atom in the ring) 4) The molecule has 4n+2 π electrons (n=0 or any positive integer Why 4n+2π Electrons? According to Hückel's Molecular Orbital Theory, a compound is particularly stable if all of its bonding molecular orbitals are filled with paired electrons. - This is true of aromatic compounds, meaning they are quite stable. - With aromatic compounds, 2 electrons fill the lowest energy molecular orbital, and 4 electrons fill each subsequent energy level (the number of subsequent energy levels is denoted by n), leaving all bonding orbitals filled and no anti-bonding orbitals occupied. This gives a total of 4n+2π electrons. - As for example: Benzene has 6π electrons. Its first 2π electrons fill the lowest energy orbital, and it has 4π electrons remaining. These 4 fill in the orbitals of the succeeding energy level. The criteria for Antiaromaticity are as follows: 1) The molecule must be cyclic and completely conjugated 2) The molecule must be planar. -

The Protection of Frequencies for Radio Astronomy 1
JOURNAL OF RESEARCH of the National Bureau of Standards-D. Radio Propagation Vol. 67D, No. 2, March- April 1963 b The Protection of Frequencies for Radio Astronomy 1 R. 1. Smith-Rose President, International Scientific Radio Union (R eceived November 5, 1962) The International T elecommunications Union in its Geneva, 1959 R adio R egulations r recognises the Radio Astronomy Service in t he two following definitions: N o. 74 Radio A st1" onomy: Astronomy based on t he reception of waves of cos mi c origin. No. 75 R adio A st1"onomy Se1"vice: A service involving the use of radio astronomy. This service differs, however, from other r adio services in two important respects. 1. It does not itself originate any radio waves, and therefore causes no interference to any other service. L 2. A large proportion of its activity is conducted by the use of reception techniques which are several orders of magnit ude )]).ore sensitive than those used in other ra dio services. In order to pursue his scientific r esearch successfully, t he radio astronomer seeks pro tection from interference first, in a number of bands of frequencies distributed t hroughout I t he s p ~ct run:; and secondly:. 1~10r e complete and s p ec i~c prote.ction fOl: t he exact frequency bands III whIch natural radIatIOn from, or absorptIOn lD, cosmIc gases IS known or expected to occur. The International R egulations referred to above give an exclusive all ocation to one freq uency band only- the emission line of h ydrogen at 1400 to 1427 Mc/s. -

Bibliography on World Conflict and Peace
DOCUMENT RESUME ED 097 246 SO 007 806 AUTHOR Boulding, Elise; Passions, J. Robert TITLE Bibliography on World Conflict and Peace. INSTITUTION American Sociological Association, Washington, D.C.; Consortium on Peace Research, Education, and Development, Boulder, Colo. PUB DATE Aug 74 NOT? 82p. AVAILABLE FROMBibliography Project, c/o Dorothy Carson, Institute of Behavioral Science, University of Colorado, Boulder, Colorado 80302 ($2.50; make checks payable to Boulding Projects Fund) EDRS PRICE MF-$0.75 BC Not Available from !DRS. PLUS POSTAGE DESCRIPTORS Bibliographies; *Conflict Resolution; Development; Disarmament; Environment; *Futures (of Society); *Global Approach; Instructional Materials; International Education; international Law; International Organizations; *Peace; Political Science; Social Action; Systems Approach; *World Affairs IDENTIFIERS *Nonviolence ABSTRACT This bibliography is compiled primarily in response to the needs of teachers and students in the new field of conflict and peace studies, defined as the analysis of the characteristics of the total world social system which make peace more probable. The introduction includes some suggestions on how to use the bibliography, sources of literature on war/peace studies, and a request to users for criticisms and suggestions. Books, monographs, research reports, journal articles, or educational materials were included when they were:(1) related to conflict management at every social level,(2) relevant to nonviolence, and (3) classic statements in an academic specialization, such as foreign policy studies when of particular significance for conflict studies. A subject guide to the main categories of the bibliography lists 18 major topics with various numbered subdivisions. Th%. main body of the bibliography lists citations by author and keys this to the topic subdivisions. -

Chem 22 Homework Set 12 1. Naphthalene Is Colorless, Tetracene
Chem 22 Homework set 12 1. Naphthalene is colorless, tetracene is orange, and azulene is blue. naphthalene tetracene azulene (a) Based on the colors observed for tetracene and azulene, what color or light does each compound absorb? (b) About what wavelength ranges do these colors correspond to? (c) Naphthalene has a conjugated π-system, so we know it must absorb somewhere in the UV- vis region of the EM spectrum. Where does it absorb? (d) What types of transitions are responsible for the absorptions? (e) Based on the absorption wavelengths, which cmpd has the smallest HOMO-LUMO gap? (f) How do you account for the difference in absorption λs of naphthalene vs tetracene? (g) Thinking about the factors that affect the absorption wavelengths, why does azulene not seem to follow the trend seen with the first two hydrocarbons? (h) Use the Rauk Hückelator (www.chem.ucalgary.ca/SHMO/) to determine the HOMO-LUMO gaps of each compound in β units. The use of this program will be demonstrated during Monday's class. 2. (a) What are the Hückel HOMO-LUMO gaps (in units of β) for the following molecules? Remember that we need to focus just on the π-systems. Use the Rauk Hückelator. (b) Use the Rauk Hückelator to draw some conjugated polyenes — linear as well as branched. Look at the HOMO. What is the correlation between the phases (ignore the sizes) of the p- orbitals that make up the HOMO and the positions of the double- and single-bonds in the Lewis structure? What is the relationship between the phases of p-orbitals of the LUMO to those of the HOMO? (c) Use your answer from part b and the pairing theorem to sketch the HOMO and LUMO of the polyenes below (again, just the phases — don't worry about the relative sizes of the p- orbitals). -

Iridium-Catalyzed Borylation of Arenes and Heteroarenes Via C–H Activation*
Pure Appl. Chem., Vol. 78, No. 7, pp. 1369–1375, 2006. doi:10.1351/pac200678071369 © 2006 IUPAC Iridium-catalyzed borylation of arenes and heteroarenes via C–H activation* Tatsuo Ishiyama and Norio Miyaura‡ Division of Chemical Process Engineering, Graduate School of Engineering, Hokkaido University, Sapporo 060-8628, Japan Abstract: Direct C–H borylation of aromatic compounds catalyzed by a transition-metal complex was studied as an economical protocol for the synthesis of aromatic boron deriva- tives. Iridium complexes generated from Ir(I) precursors and 2,2'-bipyridine ligands effi- ciently catalyzed the reactions of arenes and heteroarenes with bis(pinacolato)diboron or pinacolborane to produce a variety of aryl- and heteroarylboron compounds. The catalytic cycle involves the formation of a tris(boryl)iridium(III) species and its oxidative addition to an aromatic C–H bond. Keywords: iridium catalyst; arylboron compounds; C–H activation; pinacolborane; bis(pina- colato)diboron. INTRODUCTION Aromatic boron derivatives are an important class of compounds, the utility of which has been amply demonstrated in various fields of chemistry. Traditional methods for their synthesis are based on the re- actions of trialkylborates with aromatic lithium or magnesium reagents derived from aromatic halides [1]. Pd-catalyzed cross-coupling of aromatic halides with tetra(alkoxo)diborons or di(alkoxo)boranes is a milder variant where the preparation of magnesium and lithium reagents is avoided [2,3]. Alternatively, transition-metal-catalyzed aromatic C–H borylation of aromatic compounds by pina- colborane (HBpin, pin = O2C2Me4) or bis(pinacolato)diboron (B2pin2) is highly attractive as a con- venient, economical, and environmentally benign process for the synthesis of aromatic boron com- pounds without any halogenated reactant, which has been studied extensively by Hartwig, Marder, and η4 Smith [4–6]. -

Azulene—A Bright Core for Sensing and Imaging
molecules Review Azulene—A Bright Core for Sensing and Imaging Lloyd C. Murfin * and Simon E. Lewis Department of Chemistry, University of Bath, Bath BA2 7AY, UK; [email protected] * Correspondence: lloyd.murfi[email protected] Abstract: Azulene is a hydrocarbon isomer of naphthalene known for its unusual colour and fluores- cence properties. Through the harnessing of these properties, the literature has been enriched with a series of chemical sensors and dosimeters with distinct colorimetric and fluorescence responses. This review focuses specifically on the latter of these phenomena. The review is subdivided into two sec- tions. Section one discusses turn-on fluorescent sensors employing azulene, for which the literature is dominated by examples of the unusual phenomenon of azulene protonation-dependent fluorescence. Section two focuses on fluorescent azulenes that have been used in the context of biological sensing and imaging. To aid the reader, the azulene skeleton is highlighted in blue in each compound. Keywords: fluorescence; azulene; sensor; dosimeter; bioimaging; chemosensor; chemodosimeter 1. Introduction Azulene, 1, is an isomer of naphthalene, 2, composed of fused 5- and 7-membered ring systems (Figure1) and named for its vibrant blue colour. Unlike naphthalene, azulene is a non-alternant hydrocarbon, possessing nodal points at C-2 and C-6 of the HOMO and C-1 and C-3 of the LUMO [1]. The location of these nodes results in low electronic repulsion in the S1 singlet excited state, affording a relatively small HOMO-LUMO gap. Hence, the S0!S1 transition arises from absorption in the visible region. Conversely, in naphthalene, coefficient magnitudes remain consistent for each position in both the HOMO Citation: Murfin, L.C.; Lewis, S.E. -

Supek's Worry Ministry At
530 Nature Vol. 288 II December 1980 lines, a small majonty for recording victims of this process was the Agricultural research research carried out under P2 containment Encyclopedia Moderna, a philosophy of conditions and above, and a larger science journal edited by Dr Supek himself. majority for keeping a record merely of all Dr Supek stresses that it is not the "self Ministry at top research in P3 and P4 containment management" process - Yugoslavia's An impending change in the relationship conditions, the two strictest categories. special contribution to socialism - which between the Agricultural Research Council Reflecting their general belief that is at fault. If the current trend towards and the Ministry of Agriculture, Fisheries recombinant DNA research no longer bureaucratic centralism could be reversed, and Food (MAFF) now seems likely. Most represents a greater hazard than ordinary he said, and "self-management" restored probably, the council will in future be more research with microorganisms, many to scientists, both basic and applied directly subject to the ministry. In this committee members were sceptical about research would benefit. At present, respect, it is likely to be worse off than the the value of a broad study of the however, self-management is simply a Medical Research Council, which in effectiveness of IBCs which NIH is now slogan. October reached an arrangement with its preparing. Such official duplicity, said Dr Supek, is chief sponsoring department of If the committees had any value, it was nothing new in Yugoslav science. In 1956, government, the Department of Health felt, it had been in calming public fears when Yugoslavia began a nuclear research and Social Security, that some £12 million about the health implications of such programme, Dr Supek, as director of the of "Rothschild money" should be research. -

What Are Cosmic Rays?!
WhatWWhatWhhaatt areaarearree CosmicCCosmicCoossmmiicc Rays?!RRays?!Raayyss??!! By Hayanon Translated by Y. Noda and Y. Kamide Supervised by Y. Muraki ᵶᵶᵋᵰᵿᶗᶑᵘᴾᴾᵱᶇᶀᶊᶇᶌᶅᶑᴾᶍᶄᴾᵡᶍᶑᶋᶇᶁᴾᵰᵿᶗᶑᵋᵰᵿᶗᶑᵘᵘᴾᴾᵱᶇᶀᶊᶊᶇᶌᶅᶑᴾᶍᶄᴾᵡᶍᶑᶋᶇᶁᴾᵰᵿᶗᶑ Have you ever had an X-ray examination Scientists identified three types at the hospital? In 1896, a German of radiation: positively-charged alpha physicist, W. C. Röntgen, astonished people particles, negatively-charged beta particles, with an image of bones captured through and uncharged gamma rays. In 1903, M. the use of X-rays. He had just discovered Curie along with her husband, P. Curie, and the new type of rays emitted by a discharge Becquerel, won the Nobel prize in physics. device. He named them X-rays. Because Furthermore, M. Curie was awarded the of their high penetration ability, they are Nobel prize in chemistry in 1911. able to pass through flesh. Soon after, it Certain types of radiation including was found that excessive use of X-rays can X-rays are now used for many medical cause harm to bodies. purposes including examining inside the In that same year, a French scientist, body, treating cancer, and more. Radiation, A. H. Becquerel, found that a uranium however, could be harmful unless the compound also gave off mysterious rays. amount of radiation exposure is strictly To his surprise, they could penetrate controlled. wrapping paper and expose a photographic The work with radium by M. Curie later film generating an image of the uranium led to the breakthrough discovery of compound. The uranium rays had similar the radiation coming from space. These characteristics as those of X-rays, but were cosmic rays were discovered by an Austrian determined to be different from them.