Chorion Characteristics of Sod Webworm Eggs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moths Light a Way? by John Pickering, Tori Staples and Rebecca Walcott
SOUTHERN LEPIDOPTERISTS NEWS VOLUME 38 NO4. (2016), PG. 331 SAVE ALL SPECIES – MOTHS LIGHT A WAY? BY JOHN PICKERING, TORI STAPLES AND REBECCA WALCOTT Abstract -- What would it take to save all species from snakes, and stinging insects, they pose no health risk. extinction? A new initiative, Save all species, plans to Moths are an exceedingly species-rich group, for which answer this question and provide the tools we need to do the diversity at a terrestrial site will typically exceed any so by 2050. Here we consider the merits and problems other taxon except for beetles. Because moth larvae are associated with inventorying moths to help decide which restricted in their diet to specific host taxa, differences in terrestrial areas to protect. We compare the the assemblages of resident moth species could reflect scientifically-described moth fauna of the British Isles differences across sites in plants and other hosts. If which, with 2,441 species, is taxonomically complete, that’s true, we may be able to use moth inventories as with 11,806 described species from North America north efficient proxies to compare surrounding plant of Mexico, the fauna of which is not fully described. As communities. a percentage of the described moth fauna, there are fewer “macro” moths (Geometroidea, Drepanoidea, Inventorying moths presents challenges, notably, Noctuoidea, Bombycoidea, Lasiocampidae) in the sampling smaller species, describing thousands of British Isles (34.9%) than those known for the United species new to science, and identifying specimens States and Canada (46.1%). We present data on 1,254 accurately. Our experience is that we can identify 99% species for an intensively-studied site in Clarke County, of moths from digital images to species, species-groups, Georgia and consider whether species in the British Isles which contain species of similar appearance, or are generally smaller than ones in Georgia. -

Larger Sod Webworm
Pest Profile Photo credit: Jessica Louque, Smithers Viscient, Bugwood.org licensed under a Creative Commons Attribution 3.0 License. Common Name: Large Sod Webworm or Greater Sod Webworm Scientific Name: Pediasia trisecta Order and Family: Lepidoptera; Crambidae Size and Appearance: Length (mm) Appearance Egg - Tiny, oval-shaped - Have longitudinal ribbing on surface Larva/Nymph - Beige, gray, light brown or greenish in color - Generally have spots on their abdomen - Have paired dorsal and lateral spots on each 16-25 mm abdominal segment - The head is yellowish-brown, brown, or black in color Adult - Forewings are brown to pale yellowish-orange and light gray on the lower half -Hindwing is broader than forewing and grayish-white Wingspan: 23-33 mm in color with whitish fringe - Wings are covered in checkered pattern scales - Wings are rolled around body when at rest - Have a “snout” Pupa (if applicable) - Pupation occurs underground - Torpedo-shaped cocoon is made of silk mixed with 10-13 mm bits of plants and soil - Tan to dark brown in color Type of feeder (Chewing, sucking, etc.): Larvae have chewing mouthparts. Host plant/s: Bluegrass, bentgrass, ryegrass, and fescues. Description of Damage (larvae and adults): All damage is done by larval feeding. Young larvae will be located within leaf folds at the base of the plant where they will feed on the leaf surface. As they mature they will move to the base of the plant where they will form a silken tube that they will retreat into during the hot summer days. Larval feeding can occur on the upper root system, stems, and blades of grass. -
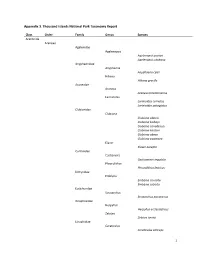
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Lepidoptera: Crambidae, Crambinae) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Li, W. C.; Li, H. H. One new species of the genus Pediasia Hübner, [1825] from China (Lepidoptera: Crambidae, Crambinae) SHILAP Revista de Lepidopterología, vol. 39, núm. 154, junio, 2011, pp. 235-239 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Disponible en: http://www.redalyc.org/articulo.oa?id=45521389010 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto 235-239 One new species of the 10/6/11 11:33 Página 235 SHILAP Revta. lepid., 39 (154), junio 2011: 235-239 CODEN: SRLPEF ISSN:0300-5267 One new species of the genus Pediasia Hübner, [1825] from China (Lepidoptera: Crambidae, Crambinae) W. C. Li & H. H. Li Abstract Pediasia rotundiprojecta Li & Li, sp. n. is described from Tibet, China. The female of Pediasia pseudopersella Bleszyn´ski, 1959 is described for the first time. Image of adult of the new species is provided and the genitalia are illustrated. KEY WORDS: Lepidoptera, Crambidae, Crambinae, Pediasia, new species, China. Una nueva especie de China del género Pediasia Hübner, [1825] (Lepidoptera: Crambidae, Crambinae) Resumen Se describe del Tibet, China Pediasia rotundiprojecta Li & Li, sp. n. Se describe por primera vez la hembra de Pediasia pseudopersella Bleszyn´ski, 1959. Se proporciona la imagen del adulto de la nueva especie y se ilustra su genitalia. -

Journal of the Lepidopterists' Society
JOURNAL OF THE LEPIDOPTERISTS' SOCIETY Volume 38 1984 Number 3 Joumal of the Lepidopterists' Society 38(3). 1984. 149-164 SOD WEBWORM MOTHS (PYRALIDAE: CRAMBINAE) IN SOUTH DAKOTA B. McDANIEL,l G. FAUSKEl AND R. D. GUSTIN 2 ABSTRACT. Twenty-seven species of the subfamily Crambinae known as sod web worm moths were collected from South Dakota. A key to species has been included as well as their distribution patterns in South Dakota. This study began after damage to rangeland in several South Dakota counties in the years 1974 and 1975. Damage was reported from Cor son, Dewey, Harding, Haakon, Meade, Perkins, Stanley and Ziebach counties. An effort was made to determine the species of Crambinae present in South Dakota and their distribution. Included are a key for species identification and a list of species with their flight periods and collection sites. MATERIALS AND METHODS Black light traps using the General Electric Fluorescent F ls T8 B1 15 watt bulb were set up in Brookings, Jackson, Lawrence, Minnehaha, Pennington and Spink counties. In Minnehaha County collecting was carried out with a General Electric 200 watt soft-glow bulb. Daytime collecting was used in several localities. Material in the South Dakota State University Collection was also utilized. For each species a map is included showing collection localities by county. On the maps the following symbols are used: • = collected by sweepnet. Q = collected by light trap. Key to South Dakota Cram binae 1a. Rs stalked .__ ... ___ .. __ ......................... _..... _ ................................. _._............................................. 2 lb. Rs arising directly from discal cell ................................................................. _............ _............ -

Developing Biodiverse Green Roofs for Japan: Arthropod and Colonizer Plant Diversity on Harappa and Biotope Roofs
20182018 Green RoofsUrban and Naturalist Urban Biodiversity SpecialSpecial Issue No. Issue 1:16–38 No. 1 A. Nagase, Y. Yamada, T. Aoki, and M. Nomura URBAN NATURALIST Developing Biodiverse Green Roofs for Japan: Arthropod and Colonizer Plant Diversity on Harappa and Biotope Roofs Ayako Nagase1,*, Yoriyuki Yamada2, Tadataka Aoki2, and Masashi Nomura3 Abstract - Urban biodiversity is an important ecological goal that drives green-roof in- stallation. We studied 2 kinds of green roofs designed to optimize biodiversity benefits: the Harappa (extensive) roof and the Biotope (intensive) roof. The Harappa roof mimics vacant-lot vegetation. It is relatively inexpensive, is made from recycled materials, and features community participation in the processes of design, construction, and mainte- nance. The Biotope roof includes mainly native and host plant species for arthropods, as well as water features and stones to create a wide range of habitats. This study is the first to showcase the Harappa roof and to compare biodiversity on Harappa and Biotope roofs. Arthropod species richness was significantly greater on the Biotope roof. The Harappa roof had dynamic seasonal changes in vegetation and mainly provided habitats for grassland fauna. In contrast, the Biotope roof provided stable habitats for various arthropods. Herein, we outline a set of testable hypotheses for future comparison of these different types of green roofs aimed at supporting urban biodiversity. Introduction Rapid urban growth and associated anthropogenic environmental change have been identified as major threats to biodiversity at a global scale (Grimm et al. 2008, Güneralp and Seto 2013). Green roofs can partially compensate for the loss of green areas by replacing impervious rooftop surfaces and thus, contribute to urban biodiversity (Brenneisen 2006). -

Great Lakes Entomologist
- Vol. 3'3, No.3 & 4 Fall/Winter 2000 THE GREAT LAKES ENTOMOLOGIST PUBLISHED BY THE MICHIGAN ENTOMOLOGICAL SOCIETY THE GREAT LAKES ENTOMOLOGIST Published by the Michigan Entomological Society Volume 33 No. 3-4 ISSN 0090-0222 TABLE OF CONTENTS New distribution record for the endangered crawling water beetle, Brychius hungerfordi (Coleoptera: Haliplidael ond notes on seasonal abundance and food preferences Michael Grant, Robert Vande Kopple and Bert Ebbers . ............... 165 Arhyssus hirtus in Minnesota: The inland occurrence of an east coast species (Hemiptera: Heteroptera: RhopalidaeJ John D. Laftin and John Haarstad .. 169 Seasonol occurrence of the sod webworm moths (lepidoptera: Crambidoe) of Ohio Harry D. Niemczk, David 1. Shetlar, Kevin T. Power and Douglas S. Richmond. ... 173 Leucanthiza dircella (lepidoptera: Gracillariidae): A leafminer of leatherwood, Dirca pa/us/tis Toby R. Petriee, Robert A Haack, William J. Mattson and Bruce A. Birr. 187 New distribution records of ground beetles from the north-central United States (Coleoptera: Carabidae) Foster Forbes Purrington, Daniel K. Young, Kirk 1. lorsen and Jana Chin-Ting lee .......... 199 Libel/ula flavida (Odonata: libellulidoe], a dragonfly new to Ohio Tom D. Schultz. ... 205 Distribution of first instar gypsy moths [lepidoptera: Lymantriidae] among saplings of common Great Lakes understory species 1. l. and J. A. Witter ....... 209 Comparison of two population sampling methods used in field life history studies of Mesovelia mulsonti (Heteroptera: Gerromorpha: Mesoveliidae) in southern Illinois Steven J. Taylor and J. E McPherson ..... .. 223 COVER PHOTO Scudderio furcata Brunner von Wattenwyl (Tettigoniidae], the forktailed bush katydid. Photo taken in Huron Mountains, MI by M. F. O'Brien. -

Sod Webworm Pest Fact Sheet 48 Dr
Bringing information and education into the communities of the Granite State Sod Webworm Pest Fact Sheet 48 Dr. Stanley R. Swier, Extension Specialist Emeritus, Entomology Introduction and Description Sod webworms are the caterpillar stage of small moths, from the genera Crambus and Herpetogramma. Adult moths are about 1" long, tan-colored, and are often seen flying about the lawn in jerky, short flight. Full-grown larvae (caterpillars) are 3/4" long, brown or gray with spots. The larvae construct silk-lined tunnels in soil or thatch, but come out to the surface at night to feed on grass. Life Cycle Spring: When the weather warms up, the overwintering Adult sod webworm (snout moth, Crambus saltuellus). Credit: David Cappaert, Bugwood.org. caterpillars resume feeding on grass. Damage is first observed in late-June or July. Summer: Adults or moths appear in July, mate, and lay eggs. The larvae of these moths then cause damage in August. Birds make probing holes into the turf Fall: Adults appear again in September, mate, and lay eggs. The as they search for caterpillars, which larvae of these eggs overwinter in the soil. can cause significant damage. Damage The damage caused by sod webworms shows up first as small, irregular brown patches. Flocks of birds seen on the turf are a good indicator of the presence of sod webworms. Birds make probing holes into the turf as they search for caterpillars, which can cause significant damage. Management IPM Strategies: • Monitoring - To sample for sod webworms, look carefully at the damaged area for silken tunnels. Alternatively, mix Immature sod webworm. -

Striped Sod Wej^Worm, Crambus Mutabius Clemens1
STRIPED SOD WEJ^WORM, CRAMBUS MUTABIUS CLEMENS1 By GKORGE G. AINSUö Entomological Assistant, Cereal and Forage Insect Investigationst Bureau of Entorno fogy, United States Department of Agriculture INTRODUCTION Throughout a wide area Crambus mutabilis is one of the most common species of the genus. It ranks well toward the head of the list in de- structiveness, although by itself it never has been directly charged with a destructive outbreak. It has not previously received detailed study, and the available information concerning it is scattered and meager. The present paper includes a summary of previously published facts, together with the results of the writer's studies for several years. SYSTEMATIC HISTORY Cr ambus mutabilis was first described by Clemens (j, />. 204)2 in i860, but he furnished no information as to the source of his material. Three years later Zeller (15, p. 44) redescribed it as Crambus fuscicostellus, a name which better characterizes the species than Clemens's adjective. Both names appear in the literature for some years, although Grote (7, p. 79) early recognized their probable synonymy. Smith ( 13, p. 87) first placed fuscicostettus unconditionally as a synonyjn of mutabilis, in which he is fully borne out by Hampton {8y p. 928), who haçi Zeller's type in the British Museum for comparison. GEOGRAPHICAL DISTRIBUTION Crambus mutabilis seevçis to be ^purely North American spçdes, for out- side of North Amgrica it ^as ^qen reported only by Hedemann, (9, p. joo), from St. Thomajs Island in the ^fest Inclies It is widespr^d over the pastern half of $he United States. -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

An Annotated List of the Lepidoptera of Alberta, Canada
A peer-reviewed open-access journal ZooKeys 38: 1–549 (2010) Annotated list of the Lepidoptera of Alberta, Canada 1 doi: 10.3897/zookeys.38.383 MONOGRAPH www.pensoftonline.net/zookeys Launched to accelerate biodiversity research An annotated list of the Lepidoptera of Alberta, Canada Gregory R. Pohl1, Gary G. Anweiler2, B. Christian Schmidt3, Norbert G. Kondla4 1 Editor-in-chief, co-author of introduction, and author of micromoths portions. Natural Resources Canada, Northern Forestry Centre, 5320 - 122 St., Edmonton, Alberta, Canada T6H 3S5 2 Co-author of macromoths portions. University of Alberta, E.H. Strickland Entomological Museum, Department of Biological Sciences, Edmonton, Alberta, Canada T6G 2E3 3 Co-author of introduction and macromoths portions. Canadian Food Inspection Agency, Canadian National Collection of Insects, Arachnids and Nematodes, K.W. Neatby Bldg., 960 Carling Ave., Ottawa, Ontario, Canada K1A 0C6 4 Author of butterfl ies portions. 242-6220 – 17 Ave. SE, Calgary, Alberta, Canada T2A 0W6 Corresponding authors: Gregory R. Pohl ([email protected]), Gary G. Anweiler ([email protected]), B. Christian Schmidt ([email protected]), Norbert G. Kondla ([email protected]) Academic editor: Donald Lafontaine | Received 11 January 2010 | Accepted 7 February 2010 | Published 5 March 2010 Citation: Pohl GR, Anweiler GG, Schmidt BC, Kondla NG (2010) An annotated list of the Lepidoptera of Alberta, Canada. ZooKeys 38: 1–549. doi: 10.3897/zookeys.38.383 Abstract Th is checklist documents the 2367 Lepidoptera species reported to occur in the province of Alberta, Can- ada, based on examination of the major public insect collections in Alberta and the Canadian National Collection of Insects, Arachnids and Nematodes.