Activity 1.1: Understanding the Greenhouse Effect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Greenhouse Gas Emissions from Public Lands
The Climate Report 2020: Greenhouse Gas Emissions from Public Lands As the world works to respond to the dire regulations meant to protect the public and the warnings issued last fall by the United Nations environment from these exact decisions. New Environment Programme, the Trump policies have made it cheaper and easier for administration continues to open up as much fossil energy corporations to gain and hold of our shared public lands as possible to fossil control of public lands. And they have hidden fuel extraction.1 And while doing so, the federal from public view the implications of these government continues to keep the public (aka decisions for taxpayers and the planet. the owners of these resources) in the dark on This report seeks to pull the curtain back on the mounting greenhouse gas emissions that this situation and shed light on the range of would result from drilling on our public lands. potential climate consequences of these At the same time, we’ve seen this leasing decisions. administration water down policies and 1 UN. 26 Nov. 2019. stories/press-release/cut-global-emissions-76- https://www.unenvironment.org/news-and- percent-every-year-next-decade-meet-15degc Key Takeaways: The federal government cannot manage development of these leases could be as what it does not measure, yet the Trump high as 6.6 billion MT CO2e. administration is actively seeking to These leasing decisions have significant suppress disclosure of climate emissions and long-term ramifications for our climate from fossil energy leases on public lands. and our ability to stave off the worst The leases issued during the Trump impacts of global warming. -

Climate Change and Human Health: Risks and Responses
Climate change and human health RISKS AND RESPONSES Editors A.J. McMichael The Australian National University, Canberra, Australia D.H. Campbell-Lendrum London School of Hygiene and Tropical Medicine, London, United Kingdom C.F. Corvalán World Health Organization, Geneva, Switzerland K.L. Ebi World Health Organization Regional Office for Europe, European Centre for Environment and Health, Rome, Italy A.K. Githeko Kenya Medical Research Institute, Kisumu, Kenya J.D. Scheraga US Environmental Protection Agency, Washington, DC, USA A. Woodward University of Otago, Wellington, New Zealand WORLD HEALTH ORGANIZATION GENEVA 2003 WHO Library Cataloguing-in-Publication Data Climate change and human health : risks and responses / editors : A. J. McMichael . [et al.] 1.Climate 2.Greenhouse effect 3.Natural disasters 4.Disease transmission 5.Ultraviolet rays—adverse effects 6.Risk assessment I.McMichael, Anthony J. ISBN 92 4 156248 X (NLM classification: WA 30) ©World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dis- semination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications—whether for sale or for noncommercial distribution—should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Statement Climate Change Greenhouse Effect
Western Region Technical Attachment No. 91-07 February 19, 1991 STATEMENT ON CLIMATE. CHANGE AND THE GREENHOUSE EFFECT STATEMENT ON CLIMATE CHANGE AND THE GREENHOUSE EFFECT by REGIONAL CLIMATE CENTERS High Plains Climate Center· University of Nebraska Midwestern Climate Center • Illinois State Water Survey Northeast Regional Climate Center - Cornell University Southern Regional Climate Center • Louisiana State University Southeastern Regional Climate Center • South Carolina Water Commission "'estern Regional Climate Center - Desert Research Institute March 1990 STATEMENT ON CLIMATE CHANGE AND THE GREENHOUSE EFFECT by Regional Climate Centers The Issue Many scientists have issued claims of future global climate changes towards warmer conditions as a result of the ever increasing global release of Carbon Dioxide (C02) and other trace gases from the burning of fossil fuels and from deforestation. The nation experienced an unexpected and severe drought in 1988 which continued through 1989 in parts of the western United States.Is there a connection between these two atmospheric issues? Was the highly unusual 1988-89 drought the first symptom of the climate change atmospheric scientists had been talking about for the past 10 years? Most of the scientific community say "no." The 1988 drought was probably not tied to the ever increasing atmospheric burden of our waste gases. The 1988 drought fits within the historical range of climatic extremes over the past 100 years. Regardless, global climate. change due to the greenhouse effect is an issue of growing national and international concern. It joins the acid rain and ozone layer issues as major atmospheric problems arising primarily from human activities. The term "Greenhouse Effect" derives from the loose analogy between the behavior of the absorbing trace gases in the atmosphere and the window glass in a greenhouse. -

Agriculture, Forestry, and Other Human Activities
4 Agriculture, Forestry, and Other Human Activities CO-CHAIRS D. Kupfer (Germany, Fed. Rep.) R. Karimanzira (Zimbabwe) CONTENTS AGRICULTURE, FORESTRY, AND OTHER HUMAN ACTIVITIES EXECUTIVE SUMMARY 77 4.1 INTRODUCTION 85 4.2 FOREST RESPONSE STRATEGIES 87 4.2.1 Special Issues on Boreal Forests 90 4.2.1.1 Introduction 90 4.2.1.2 Carbon Sinks of the Boreal Region 90 4.2.1.3 Consequences of Climate Change on Emissions 90 4.2.1.4 Possibilities to Refix Carbon Dioxide: A Case Study 91 4.2.1.5 Measures and Policy Options 91 4.2.1.5.1 Forest Protection 92 4.2.1.5.2 Forest Management 92 4.2.1.5.3 End Uses and Biomass Conversion 92 4.2.2 Special Issues on Temperate Forests 92 4.2.2.1 Greenhouse Gas Emissions from Temperate Forests 92 4.2.2.2 Global Warming: Impacts and Effects on Temperate Forests 93 4.2.2.3 Costs of Forestry Countermeasures 93 4.2.2.4 Constraints on Forestry Measures 94 4.2.3 Special Issues on Tropical Forests 94 4.2.3.1 Introduction to Tropical Deforestation and Climatic Concerns 94 4.2.3.2 Forest Carbon Pools and Forest Cover Statistics 94 4.2.3.3 Estimates of Current Rates of Forest Loss 94 4.2.3.4 Patterns and Causes of Deforestation 95 4.2.3.5 Estimates of Current Emissions from Forest Land Clearing 97 4.2.3.6 Estimates of Future Forest Loss and Emissions 98 4.2.3.7 Strategies to Reduce Emissions: Types of Response Options 99 4.2.3.8 Policy Options 103 75 76 IPCC RESPONSE STRATEGIES WORKING GROUP REPORTS 4.3 AGRICULTURE RESPONSE STRATEGIES 105 4.3.1 Summary of Agricultural Emissions of Greenhouse Gases 105 4.3.2 Measures and -

ALBEDO ENHANCEMENT by STRATOSPHERIC SULFUR INJECTIONS: a CONTRIBUTION to RESOLVE a POLICY DILEMMA? an Editorial Essay
ALBEDO ENHANCEMENT BY STRATOSPHERIC SULFUR INJECTIONS: A CONTRIBUTION TO RESOLVE A POLICY DILEMMA? An Editorial Essay Fossil fuel burning releases about 25 Pg of CO2 per year into the atmosphere, which leads to global warming (Prentice et al., 2001). However, it also emits 55 Tg S as SO2 per year (Stern, 2005), about half of which is converted to sub-micrometer size sulfate particles, the remainder being dry deposited. Recent research has shown that the warming of earth by the increasing concentrations of CO2 and other greenhouse gases is partially countered by some backscattering to space of solar radiation by the sulfate particles, which act as cloud condensation nuclei and thereby influ- ence the micro-physical and optical properties of clouds, affecting regional precip- itation patterns, and increasing cloud albedo (e.g., Rosenfeld, 2000; Ramanathan et al., 2001; Ramaswamy et al., 2001). Anthropogenically enhanced sulfate particle concentrations thus cool the planet, offsetting an uncertain fraction of the anthro- pogenic increase in greenhouse gas warming. However, this fortunate coincidence is “bought” at a substantial price. According to the World Health Organization, the pollution particles affect health and lead to more than 500,000 premature deaths per year worldwide (Nel, 2005). Through acid precipitation and deposition, SO2 and sulfates also cause various kinds of ecological damage. This creates a dilemma for environmental policy makers, because the required emission reductions of SO2, and also anthropogenic organics (except black carbon), as dictated by health and ecological considerations, add to global warming and associated negative conse- quences, such as sea level rise, caused by the greenhouse gases. -

ENERGYENERCY a Balancing Act
Educational Product Educators Grades 9–12 Investigating the Climate System ENERGYENERCY A Balancing Act PROBLEM-BASED CLASSROOM MODULES Responding to National Education Standards in: English Language Arts ◆ Geography ◆ Mathematics Science ◆ Social Studies Investigating the Climate System ENERGYENERGY A Balancing Act Authored by: CONTENTS Eric Barron, College of Earth and Mineral Science, Pennsylvania Grade Levels; Time Required; Objectives; State University, University Park, Disciplines Encompassed; Key Terms; Pennsylvania Prerequisite Knowledge . 2 Prepared by: Scenario. 5 Stacey Rudolph, Senior Science Education Specialist, Institute for Part 1: Understanding the absorption of energy Global Environmental Strategies at the surface of the Earth. (IGES), Arlington, Virginia Question: Does the type of the ground surface John Theon, Former Program influence its temperature? . 5 Scientist for NASA TRMM Part 2: How a change in water phase affects Editorial Assistance, Dan Stillman, surface temperatures. Science Communications Specialist, Institute for Global Environmental Question: How important is the evaporation of Strategies (IGES), Arlington, Virginia water in cooling a surface? . 6 Graphic Design by: Part 3: Determining what controls the temperature Susie Duckworth Graphic Design & of the land surface. Illustration, Falls Church, Virginia Question 1: If my town grows, will it impact the Funded by: area’s temperature? . 7 NASA TRMM Grant #NAG5-9641 Question 2: Why are the summer temperatures in the desert southwest so much higher than at the Give us your feedback: To provide feedback on the modules same latitude in the southeast? . 8 online, go to: Appendix A: Bibliography/Resources . 9 https://ehb2.gsfc.nasa.gov/edcats/ educational_product Appendix B: Answer Keys . 10 and click on “Investigating the Appendix C: National Education Standards. -

Energy Budget: Earth’S Most Important and Least Appreciated Planetary Attribute by Lin Chambers (NASA Langley Research Center) and Katie Bethea (SSAI)
© 2013, Astronomical Society of the Pacific No. 84 • Summer 2013 www.astrosociety.org/uitc 390 Ashton Avenue, San Francisco, CA 94112 Energy Budget: Earth’s most important and least appreciated planetary attribute by Lin Chambers (NASA Langley Research Center) and Katie Bethea (SSAI) asking in the Sun on a warm day, it’s easy for know some species of animals can see ultraviolet people to realize that most of the energy on light and portions of the infrared spectrum. NASA Earth comes from the Sun; students know satellites use instruments that can “see” different Bthis as early as elementary school. We all know parts of the electromagnetic spectrum to observe plants use this energy from the Sun for photosyn- various processes in the Earth system, including the thesis, and animals eat plants, creating a giant food energy budget. web. Most people also understand the Sun’s energy The Sun is a very hot ball of plasma emitting large drives evaporation and thus powers the water cycle. amounts of energy. By the time it reaches Earth, this But many people do not realize the Sun’s energy it- energy amounts to about 340 Watts for every square self is also part of an important interconnected sys- meter of Earth on average. That’s almost 6 60-Watt tem: Earth’s energy budget or balance. This energy light bulbs for every square meter of Earth! With budget determines conditions on our planet — just all of that energy shining down on the Earth, how like the energy budget of other planets determines does our planet maintain a comfortable balance that conditions there. -

Methodology for Afforestation And
METHODOLOGY FOR THE QUANTIFICATION, MONITORING, REPORTING AND VERIFICATION OF GREENHOUSE GAS EMISSIONS REDUCTIONS AND REMOVALS FROM AFFORESTATION AND REFORESTATION OF DEGRADED LAND VERSION 1.2 May 2017 METHODOLOGY FOR THE QUANTIFICATION, MONITORING, REPORTING AND VERIFICATION OF GREENHOUSE GAS EMISSIONS REDUCTIONS AND REMOVALS FROM AFFORESTATION AND REFORESTATION OF DEGRADED LAND VERSION 1.2 May 2017 American Carbon Registry® WASHINGTON DC OFFICE c/o Winrock International 2451 Crystal Drive, Suite 700 Arlington, Virginia 22202 USA ph +1 703 302 6500 [email protected] americancarbonregistry.org ABOUT AMERICAN CARBON REGISTRY® (ACR) A leading carbon offset program founded in 1996 as the first private voluntary GHG registry in the world, ACR operates in the voluntary and regulated carbon markets. ACR has unparalleled experience in the development of environmentally rigorous, science-based offset methodolo- gies as well as operational experience in the oversight of offset project verification, registration, offset issuance and retirement reporting through its online registry system. © 2017 American Carbon Registry at Winrock International. All rights reserved. No part of this publication may be repro- duced, displayed, modified or distributed without express written permission of the American Carbon Registry. The sole permitted use of the publication is for the registration of projects on the American Carbon Registry. For requests to license the publication or any part thereof for a different use, write to the Washington DC address listed above. -

Plastic & Climate
EXECUTIVE SummarY Plastic & Climate THE HIDDEN COsts OF A PLAstIC PLANET Plastic Proliferation Threatens the Climate on a Global Scale he plastic pollution crisis that overwhelms our oceans is also a FIGURFIGUREE 1 significant and growing threat to the Earth’s climate. At current Annual Plastic Emissions to 2050 Tlevels, greenhouse gas emissions from the plastic lifecycle threaten the ability of the global community to keep global temperature rise below 3.0 billion metric tons 1.5°C. With the petrochemical and plastic industries planning a massive expansion in production, the problem is on track to get much worse. By 2050, annual emissions could grow to more than 2.5 2.75 billion metric tons Greenhouse gas emissions from the plastic lifecycle of CO2e from plastic threaten the ability of the global community to keep production and incineration. global temperature rise below 1.5°C. By 2050, the 2.0 greenhouse gas emissions from plastic could reach Annual emissions from incineration over 56 gigatons—10-13 percent of the entire 1.5 remaining carbon budget. If plastic production and use grow as currently planned, by 2030, these 1.0 emissions could reach 1.34 gigatons per year—equivalent to the emissions released by more than 295 new 500-megawatt coal-fired power plants. Annual emissions from By 2050, the cumulation of these greenhouse gas emissions from plastic 0.5 resin and fiber production could reach over 56 gigatons—10–13 percent of the entire remaining carbon budget. Nearly every piece of plastic begins as a fossil fuel, and greenhouse gases 0 are emitted at each of each stage of the plastic lifecycle: 1) fossil fuel 2015 2020 2030 2040 2050 extraction and transport, 2) plastic refining and manufacture, 3) managing plastic waste, and 4) plastic’s ongoing impact once it reaches our oceans, Source: CIEL waterways, and landscape. -

CO2 Mitigation Through Biofuels in the Transport Sector
ifeu - Institute for Energy and Environmental Research Heidelberg Germany CO2 Mitigation through Biofuels in the Transport Sector Status and Perspectives Main Report supported by FVV, Frankfurt and UFOP, Berlin CO2 Mitigation through Biofuels in the Transport Sector Status and Perspectives Main Report Heidelberg, Germany, August 2004 Authors Dr. Markus Quirin Dipl.-Phys. Ing. Sven O. Gärtner Dr. Martin Pehnt Dr. Guido A. Reinhardt This report was executed by IFEU – Institut für Energie- und Umweltforschung Heidelberg GmbH (Institute for Energy and Environmental Research Heidelberg) Wilckensstrasse 3, 69120 Heidelberg, Germany Tel: +49-6221-4767-0, Fax -19 E-Mail: [email protected] www.ifeu.de Funding organisations F V V – Research Association for Combustion Engines F V V e. V. im VDMA, Lyoner Straße 18, 60528 Frankfurt am Main, Germany www.fvv-net.de UFOP – Union for the Promotion of Oil and Protein Plants UFOP e. V., Reinhardtstraße 18, 10117 Berlin, Germany www.ufop.de FAT – German Association for Research on Automobile-Technique FAT e. V. im VDA, Westendstraße 61, 60325 Frankfurt am Main, Germany www.vda.de/de/vda/intern/organisation/abteilungen/fat.html More information on this project can be found on www.ifeu.de/co2mitigation.htm Acknowledgements We thank the Research Association for Combustion Engines (Forschungsvereinigung Verbrennungskraftmaschinen e. V., FVV) that called this study into existence. Thanks are also due to the Union for the Promotion of Oil and Protein Plants (Union zur Förderung von Oel- und Proteinpflanzen e. V., UFOP) and the German Association for Research on Automobile-Technique (Forschungsvereinigung Automobiltechnik e. V., FAT) that, together with FVV, financed this research. -
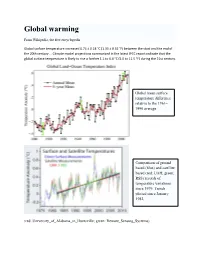
Global Warming
Global warming From Wikipedia, the free encyclopedia Global surface temperature increased 0.74 ± 0.18 °C (1.33 ± 0.32 °F) between the start and the end of the 20th century … Climate model projections summarized in the latest IPCC report indicate that the global surface temperature is likely to rise a further 1.1 to 6.4 °C (2.0 to 11.5 °F) during the 21st century. Global mean surface temperature difference relative to the 1961– 1990 average Comparison of ground based (blue) and satellite based (red: UAH; green: RSS) records of temperature variations since 1979. Trends plotted since January 1982. (red: University_of_Alabama_in_Huntsville; green: Remote_Sensing_Systems) Temperature is believed to have been relatively stable over the one or two thousand years before 1850, with regionally-varying fluctuations such as the Medieval Warm Period and the Little Ice Age. Two millennia of mea n surface temperatures according to different reconstructions, each smoothed on a decadal scale. The instrumental record and the unsmoothed annual value for 2004 are shown in black. Greenhouse gases The greenhouse effect is the process by which absorption and emission of infrared radiation by gases in the atmosphere warm a planet's lower atmosphere and surface. It was discovered by Joseph Fourier in 1824. Existence of the greenhouse effect as such is not disputed, even by those who do not agree that the recent temperature increase is attributable to human activity. The question is instead how the strength of the greenhouse effect changes when human activity increases the concentrations of greenhouse gases in the atmosphere. Naturally occurring greenhouse gases have a mean warming effect of about 33 °C (59 °F). -

Information on Selected Climate and Climate-Change Issues
INFORMATION ON SELECTED CLIMATE AND CLIMATE-CHANGE ISSUES By Harry F. Lins, Eric T. Sundquist, and Thomas A. Ager U.S. GEOLOGICAL SURVEY Open-File Report 88-718 Reston, Virginia 1988 DEPARTMENT OF THE INTERIOR DONALD PAUL MODEL, Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director For additional information Copies of this report can be write to: purchased from: Office of the Director U.S. Geological Survey U.S. Geological Survey Books and Open-File Reports Section Reston, Virginia 22092 Box 25425 Federal Center, Bldg. 810 Denver, Colorado 80225 PREFACE During the spring and summer of 1988, large parts of the Nation were severely affected by intense heat and drought. In many areas agricultural productivity was significantly reduced. These events stimulated widespread concern not only for the immediate effects of severe drought, but also for the consequences of potential climatic change during the coming decades. Congress held hearings regarding these issues, and various agencies within the Executive Branch of government began preparing plans for dealing with the drought and potential climatic change. As part of the fact-finding process, the Assistant Secretary of the Interior for Water and Science asked the Geological Survey to prepare a briefing that would include basic information on climate, weather patterns, and drought; the greenhouse effect and global warming; and climatic change. The briefing was later updated and presented to the Secretary of the Interior. The Secretary then requested the Geological Survey to organize the briefing material in text form. The material contained in this report represents the Geological Survey response to the Secretary's request.