Makena (Hydroxyprogesterone Caproate) Injection Label
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Association of Induced Abortion with Hypertensive Disorders Of
www.nature.com/scientificreports OPEN Association of induced abortion with hypertensive disorders of pregnancy risk among nulliparous women in China: a prospective cohort study Yinhua Su1,2, Xiaoping Xie3, Yanfang Zhou1, Hong Lin1, Yamei Li1, Na Feng1 & Jiayou Luo1* The relationship between induced abortion(IA) and hypertensive disorders of pregnancy(HDP) is inconclusive. Few studies have been conducted in China. In order to clarify the association between previous IA and risk of HDP, including gestational hypertension(GH) and pre-eclampsia(PE), we performed a community-based prospective cohort study enrolling 5191 eligible nulliparous women in selected 2 districts and 11 towns of Liuyang from 2013 to 2015. Multivariable logistic regression was conducted to examine whether IA was associated with HDP, GH and PE. Of the gravidea, 1378(26.5%) had a previous IA and 258(5.0%) diagnosed with HDP, including 141(2.7%) GH and 117(2.3%) PE. The diference in the incidence of GH and PE between gravidae having one versus those with two or more IAs was minimal. After adjustment for maternal age, body mass index at frst antenatal visit, education, virus infection and history of medical disorders, previous IA was signifcantly associated with HDP (OR = 0.67, 95%CI = 0.49 to 0.91) and PE (OR = 0.61, 95%CI = 0.38 to 0.97), but not with GH (OR = 0.73, 95%CI = 0.49 to 1.10). Additional adjustment for occupation, living area, anemia, gestational diabetes mellitus, psychological stress, conception climate and infant sex, multivariable analysis provided similar results. In conclusion, previous IA was associated with a lower risk of PE among nulliparous women. -

Mother's Own Milk Feeding in Preterm Newborns Admitted to the Neonatal
International Journal of Environmental Research and Public Health Article Mother’s Own Milk Feeding in Preterm Newborns Admitted to the Neonatal Intensive Care Unit or Special-Care Nursery: Obstacles, Interventions, Risk Calculation Nadja Heller, Mario Rüdiger, Vanessa Hoffmeister and Lars Mense * Department of Pediatrics, Division of Neonatology & Pediatric Intensive Care, Saxonian Center for Feto/Neonatal Health, University Hospital Carl Gustav Carus Dresden, TU Dresden, 01307 Dresden, Germany; [email protected] (N.H.); [email protected] (M.R.); [email protected] (V.H.) * Correspondence: [email protected]; Tel.: +49-351-458-3640 Abstract: Early nutrition of newborns significantly influences their long-term health. Mother’s own milk (MOM) feeding lowers the incidence of complications in preterm infants and improves long-term health. Unfortunately, prematurity raises barriers for the initiation of MOM feeding and its continuation. Mother and child are separated in most institutions, sucking and swallowing is immature, and respiratory support hinders breastfeeding. As part of a quality-improvement project, we review the published evidence on risk factors of sustained MOM feeding in preterm neonates. Modifiable factors such as timing of skin-to-skin contact, strategies of milk expression, and infant Citation: Heller, N.; Rüdiger, M.; feeding or mode of delivery have been described. Other factors such as gestational age or neonatal Hoffmeister, V.; Mense, L. Mother’s complications are unmodifiable, but their recognition allows targeted interventions to improve MOM Own Milk Feeding in Preterm feeding. All preterm newborns below 34 weeks gestational age discharged over a two-year period Newborns Admitted to the Neonatal from our large German level III neonatal center were reviewed to compare institutional data with the Intensive Care Unit or Special-Care published evidence regarding MOM feeding at discharge from hospital. -
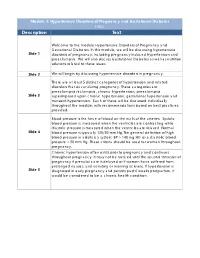
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text Welcome to the module Hypertensive Disorders of Pregnancy and Gestational Diabetes. In this module, we will be discussing hypertensive Slide 1 disorders of pregnancy, including pregnancy induced hypertension and preeclampsia. We will also discuss Gestational Diabetes as well as nutrition solutions related to these issues Slide 2 We will begin by discussing hypertensive disorders in pregnancy. There are at least 5 distinct categories of hypertension and related disorders that occur during pregnancy. These categories are: preeclampsia/eclampsia, chronic hypertension, preeclampsia Slide 3 superimposed upon chronic hypertension, gestational hypertension and transient hypertension. Each of these will be discussed individually throughout the module, with recommendations based on best practices provided. Blood pressure is the force of blood on the walls of the arteries. Systolic blood pressure is measured when the ventricles are contracting while diastolic pressure is measured when the ventricles are relaxed. Normal Slide 4 blood pressure is typically 120/80 mm Hg.The general definition of high blood pressure in adults is a systolic BP > 140 mg HG or a diastolic blood pressure > 90 mm Hg. These criteria should be used for women throughout pregnancy. Chronic hypertension often exists prior to pregnancy and continues throughout pregnancy. It may not be noticed until the second trimester of pregnancy if prenatal care is delayed or if women have suffered from prolonged nausea and vomiting or morning sickness. If hypertension is Slide 5 diagnosed in early pregnancy and persists past 6 weeks postpartum, it would be considered to be a chronic health condition. -

Causes of Newborn Death:Preterm Birth*
C AUSES OF NEWBORN DEATH: PRETERM BIRTH* Worldwide, 15 million babies are born prematurely each year. Prematurity has become the leading cause of newborn deaths worldwide, resulting in more than 1 million deaths each year.1 Preterm birth rates around the globe are increasing and are now responsible for 35% of the world’s neonatal deaths; the condition is the second-leading cause of death among children under five, after pneumonia. Babies are considered to be preterm when born alive before 37 weeks of pregnancy are completed. Over 80% of premature babies are born between 32 and 37 weeks of gestation. Most newborn deaths among this group are caused by lack of simple, essential care such as warmth and feeding support. Babies born before 28 weeks gestation require intensive care to live; however, these cases make up only 5% of preterm births. Being born preterm increases a baby’s risk of dying due to other causes, especially from neonatal infections. For preterm babies that survive, many face a lifetime of disability: preterm babies are at increased risk of cerebral palsy, learning impairment, visual disorders, and other non-communicable diseases. The burden of preterm birth is substantial for many developing countries, and scale up of some low- tech, cost-effective interventions can help to reduce newborn deaths from prematurity. Reducing the burden of preterm birth has two main elements: prevention and care. Prevention Interventions that are proven to help prevent preterm birth are clustered in the preconception, between pregnancy, and pregnancy -

The Effects of Maternal Chorioamnionitis on the Neonate
Neonatal Nursing Education Brief: The Effects of Maternal Chorioamnionitis on the Neonate https://www.seattlechildrens.org/healthcare- professionals/education/continuing-medical-nursing-education/neonatal- nursing-education-briefs/ Maternal chorioamnionitis is a common condition that can have negative effects on the neonate. The use of broad spectrum antibiotics in labor can reduce the risks, but infants exposed to chorioamnionitis continue to require treatment. The neonatal sepsis risk calculator can guide treatment. NICU, chorioamnionitis, early onset neonatal sepsis, sepsis risk calculator The Effects of Maternal Chorioamnionitis on the Neonate Purpose and Goal: CNEP # 2090 • Understand the effects of chorioamnionitis on the neonate. • Learn about a new approach for treating infants at risk. None of the planners, faculty or content specialists has any conflict of interest or will be presenting any off-label product use. This presentation has no commercial support or sponsorship, nor is it co-sponsored. Requirements for successful completion: • Successfully complete the post-test • Complete the evaluation form Date • December 2018 – December 2020 Learning Objectives • Describe the pathogenesis of maternal chorioamnionitis. • Describe the outcomes for neonates exposed to chorioamnionitis. • Identify 2 approaches for the treatment of early onset sepsis. Introduction • Chorioamnionitis is a common complication • It affects up to 10% of all pregnancies • It is an infection of the amniotic fluid and placenta • It is characterized by inflammation -

Association of Timing of Initiation of Breastmilk Expression on Milk Volume and Timing of Lactogenesis Stage II Among Mothers of Very Low-Birth-Weight Infants
BREASTFEEDING MEDICINE Volume 10, Number 2, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/bfm.2014.0089 Association of Timing of Initiation of Breastmilk Expression on Milk Volume and Timing of Lactogenesis Stage II Among Mothers of Very Low-Birth-Weight Infants Leslie A. Parker,1 Sandra Sullivan,2 Charlene Krueger,1 and Martina Mueller3 Abstract Background: Feeding breastmilk to premature infants decreases morbidity but is often limited owing to an insufficient milk supply and delayed attainment of lactogenesis stage II. Early initiation of milk expression following delivery has been shown to increase milk production in mothers of very low-birth-weight (VLBW) infants. Although recommendations for milk expression in this population include initiation within 6 hours following delivery, little evidence exists to support these guidelines. This study compared milk volume and timing of lactogenesis stage II in mothers of VLBW infants who initiated milk expression within 6 hours following delivery versus those who initiated expression after 6 hours. Subjects and Methods: Forty mothers of VLBW infants were grouped according to when they initiated milk expression following delivery. Group I began milk expression within 6 hours, and Group II began expression after 6 hours. Milk volume was measured daily for the first 7 days and on Days 21 and 42. Timing of lactogenesis stage II was determined through mothers’ perceptions of sudden breast fullness. Results: Group I produced more breastmilk during the initial expression session and on Days 6, 7, and 42. No difference in timing of lactogenesis stage II was observed. When mothers who began milk expression prior to 1 hour following delivery were removed from analysis, benefits of milk expression within 6 hours were no longer apparent. -

Practice Resource: CARE of the NEWBORN EXPOSED to SUBSTANCES DURING PREGNANCY
Care of the Newborn Exposed to Substances During Pregnancy Practice Resource for Health Care Providers November 2020 Practice Resource: CARE OF THE NEWBORN EXPOSED TO SUBSTANCES DURING PREGNANCY © 2020 Perinatal Services BC Suggested Citation: Perinatal Services BC. (November 2020). Care of the Newborn Exposed to Substances During Pregnancy: Instructional Manual. Vancouver, BC. All rights reserved. No part of this publication may be reproduced for commercial purposes without prior written permission from Perinatal Services BC. Requests for permission should be directed to: Perinatal Services BC Suite 260 1770 West 7th Avenue Vancouver, BC V6J 4Y6 T: 604-877-2121 F: 604-872-1987 [email protected] www.perinatalservicesbc.ca This manual was designed in partnership by UBC Faculty of Medicine’s Division of Continuing Professional Development (UBC CPD), Perinatal Services BC (PSBC), BC Women’s Hospital & Health Centre (BCW) and Fraser Health. Content in this manual was derived from module 3: Care of the newborn exposed to substances during pregnancy in the online module series, Perinatal Substance Use, available from https://ubccpd.ca/course/perinatal-substance-use Perinatal Services BC Care of the Newborn Exposed to Substances During Pregnancy ii Limitations of Scope Iatrogenic opioid withdrawal: Infants recovering from serious illness who received opioids and sedatives in the hospital may experience symptoms of withdrawal once the drug is discontinued or tapered too quickly. While these infants may benefit from the management strategies discussed in this module, the ESC Care Tool is intended for newborns with prenatal substance exposure. Language A note about gender and sexual orientation terminology: In this module, the terms pregnant women and pregnant individual are used. -

Evaluating Antenatal Breastmilk Expression Outcomes: a Scoping Review Imane Foudil-Bey1,2, Malia S
Foudil-Bey et al. International Breastfeeding Journal (2021) 16:25 https://doi.org/10.1186/s13006-021-00371-7 RESEARCH Open Access Evaluating antenatal breastmilk expression outcomes: a scoping review Imane Foudil-Bey1,2, Malia S. Q. Murphy1, Sandra Dunn1, Erin J. Keely3,4,5 and Darine El-Chaâr1,6,7* Abstract Background: Antenatal breastmilk expression (aBME) is recommended by some healthcare providers to improve lactation, breastfeeding, and newborn outcomes, particularly for women with diabetes as they face unique challenges with breastfeeding. However, there is limited evidence of the potential harms and benefits of this practice. Our objective was to conduct a scoping review to map the literature describing maternal and newborn outcomes of aBME. Methods: We searched Medline, Embase, CINAHL, Cochrane Database of Systematic Reviews, British Library E- Theses Online Services (EThOS) database, OpenGrey, and Clinical trials.gov from inception to January 2020. Studies in English that reported on the effect of aBME on maternal and newborn outcomes, and the experiences of women who have engaged in the practice were included for screening. Titles, abstracts, and full-text articles were screened by two independent reviewers. A critical appraisal and clinical consultation were conducted. Key findings were extracted and summarized. Results: We screened 659 studies and 20 met the inclusion criteria. The majority of included studies (n = 11, 55.0%) were published after 2015, and seven (35.0%) originated from Australia. Ten (50.0%) studies provided data on high- risk obstetrical populations, including those with diabetes (n = 8), overweight or obesity (n = 1), and preeclampsia (n = 1). Commonly reported outcomes included breastfeeding status at discharge or follow-up, mode of delivery, newborn blood glucose, and time to establishing full lactation. -

Placental Abruption in Each Phenotype of Hypertensive Disorders of Pregnancy: a Retrospective Cohort Study Using a National Inpatient Database in Japan
Hypertension Research (2021) 44:250–252 https://doi.org/10.1038/s41440-020-00557-2 COMMENT Placental abruption in each phenotype of hypertensive disorders of pregnancy: a retrospective cohort study using a national inpatient database in Japan 1 1,2 2,3 Nelson Sass ● Gilberto Nagahama ● Henri Augusto Korkes Received: 24 August 2020 / Revised: 2 September 2020 / Accepted: 3 September 2020 / Published online: 7 October 2020 © The Japanese Society of Hypertension 2020 In a very interesting paper, Naruse et al. [1] analyzed a ret- Therefore, it is plausible to claim that PE is also a “bloody rospective cohort from a Japanese database, identifying a business” (Fig. 1). higher incidence of placental abruption in women with severe Advances in the knowledge of the pathological changes preeclampsia (PE) when admitted at less than 34 weeks associated with PE, especially in the early onset forms, gestational age (GA). Abruption of the placenta is an obstetric suggest that poor trophoblastic invasion in the early stages emergency that is usually catastrophic and requires prompt triggers an inflammatory syndrome with abnormal meta- 1234567890();,: 1234567890();,: diagnosis and immediate intervention, usually by emergency bolic pathways, including placental release of large amounts cesarean section, to save the fetus and reduce the risk of antiangiogenic factors such as soluble forms-like tyrosine of bleeding complications in the mother. The results from kinase-1 (sFlt-1). High plasma levels of these factors hinder Naruse et al. [1] highlight that this serious condition has the action of vascular endothelial growth factor (VEGF) and occurred after hospital admission in expectant patients with placental growth factor (PLGF) on receptors on the endo- early onset PE at a GA less than 34 weeks. -

Pregnancy Problems in Primary Care
Moneli Golara Consultant Obstetrician and Gynaecologist Barnet Hospital 680000 babies born in the UK Pregnancy associated with change of physiology Changes results in common symptoms of pregnancy for which patients may see health care professionals Considered a ‘stress test’ on womens’ health Organ specific Skin Mucous membranes Cardiovascular Respiratory Renal GI Endocrine Specific conditions in pregnancy with cases Pigmentation Pathogenesis not completely understood Linea alba becomes nigra Areola darkens Axilla, genitalia, perineum, anus, inner thighs and neck Recent scars, freckles May take several months to recover postpartum Naevi can look suspicious during pregnancy Melasma occurs in 75% of women Oestrogen causes vascular distension and proliferation of blood vessels Spider angiomas and Naevi Mostly resolve within 3 months Palmar erythema Varicosities Venous distension of vestibule and Vagina and vulval varicosities Oedema and water retention of extremeties Purpura Striae gravidarum Hirsuitism Arms, legs back and suprapubic region Anagen and telogen hair Scalp hair appears thicker during pregnancy but hair loss and thinning 1-5 months post partum This may resolve by 15 months but never to the same state in some people Androgen alopecia involving frontal scalp may occur Blue discolouration of vagina and cervix Gingival changes, and gingivitis, bleeding ulceration and pain Hyperaemia of nasal mucosa causing congestion CO Plasma volume To encourage optimum growth of fetus Increase in red cell -

Alcohol Consumption During Pregnancy and Perinatal Results
ORIGINAL ARTICLE DOI: 10.1590/1516-3180.2015.02040211 Alcohol consumption during pregnancy and perinatal results: a cohort study Consumo de álcool durante a gravidez e resultados perinatais: um estudo de coorte Mariana SbranaI, Carlos GrandiII, Murilo BrazanI, Natacha JunqueraI, Marina Stevaux NascimentoI, Marco Antonio BarbieriIII, Heloisa BettiolIV, Viviane Cunha CardosoV Ribeirão Preto Medical School, Universidade de São Paulo (USP), Ribeirão Preto, São Paulo, Brazil ABSTRACT IMD. Medical Resident, Department of Pediatrics, CONTEXT AND OBJECTIVE: Alcohol consumption during pregnancy is a significant social problem that Ribeirão Preto Medical School, Universidade de São Paulo (USP), Ribeirão Preto, São Paulo, Brazil. may be associated with adverse perinatal outcomes. The aim of this study was to describe alcohol con- IIMD, PhD. Postdoctoral Student, Department sumption during pregnancy and to study its association with low birth weight, newborns small for gesta- of Gynecology and Obstetrics, Ribeirão Preto tional age and preterm birth. Medical School, Universidade de São Paulo DESIGN AND SETTING: Nested cohort study, in the city of Ribeirão Preto, São Paulo, Brazil. (USP), Ribeirão Preto, São Paulo, Brazil. METHODS: 1,370 women and their newborns were evaluated. A standardized questionnaire on health IIIMD, PhD. Senior Professor, Department of and lifestyle habits was applied to the mothers. Anthropometry was performed on the newborns. Alcohol Pediatrics, Ribeirão Preto Medical School, consumption was defined as low, moderate or high, as defined by the World Health Organization. -Ad Universidade de São Paulo (USP), Ribeirão Preto, justed logistic regression analysis was used. São Paulo, Brazil. RESULTS: 23% of the women consumed alcohol during pregnancy. Consumption mainly occurred in the IVMD, PhD. -

Gestational Hypertension & Pre-Eclampsia
Journal of Labor and Childbirth Gestational hypertension & pre-eclampsia Introduction • First, we talk about the definitions • When, how, why the disease occurs? • How diagnose, how to treat and how to prevent according to guidelines Moatasem Bellah Al Farrah • This lecture is to know about the disease in new way. DUMMAR Medical Center, Syria Background : Prompt identification and appropriate management of Hypertensive Disorders in Pregnancy (HDP) are essential for optimal outcomes because HDP: Biography • Are associated with severe maternal obstetric complications and increased maternal mortality risk Moatasem Bellah Al Farrah is a mem- ber of Syrian Doctors Association. He is • Lead to preterm delivery, fetal intrauterine growth restriction, low birthweight and perinatal death currently working as an assistant profes- sor in Dummar Medical center and Red Study made between 2006 and 2008 showed: 70 maternal deaths, showing leading causes of death to Crescent. He is also a member in British be: hypertension (20%), haemorrhage (19%) and embolism (17%). Chronic illness, obesity and prenatal Society for Gynecological Endoscopy, International Urogynecology Associa- risk factors were identified as important circumstances in the cases reviewed. tion, British Society for Urogynecolo- Definition and classification of Hypertensive Disorders in Pregnancy (HDP) gy. His professional interests focus on PCOS and Endometriosis Researches. Hypertensive Disorders in Pregnancy are comprised of a spectrum of disorders typically classified into categories