CMS Snapshot March 27-April 2, 2020 Delivered to You by the Partner Relations Group in the Office of Communications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

President Biden Appeals for Unity He Faces a Confluence of Crises Stemming from Pandemic, Insurrection & Race by BRIAN A
V26, N21 Thursday, Jan.21, 2021 President Biden appeals for unity He faces a confluence of crises stemming from pandemic, insurrection & race By BRIAN A. HOWEY INDIANAPOLIS – In what remains a crime scene from the insurrection on Jan. 6, President Joe Biden took the oath of office at the U.S. Capitol Wednesday, appealing to all Americans for “unity” and the survival of the planet’s oldest democ- racy. “We’ve learned again that democracy is precious,” when he declared in strongman fashion, “I alone can fix Biden said shortly before noon Wednesday after taking the it.” oath of office from Chief Justice John Roberts. “Democ- When Trump fitfully turned the reins over to Biden racy is fragile. And at this hour, my friends, democracy has without ever acknowledging the latter’s victory, it came prevailed.” after the Capitol insurrection on Jan. 6 that Senate Minor- His words of assurance came four years to the day ity Leader Mitch McConnell said he had “provoked,” leading since President Trump delivered his dystopian “American to an unprecedented second impeachment. It came with carnage” address, coming on the heels of his Republican National Convention speech in Cleveland in July 2016 Continued on page 3 Biden’s critical challenge By BRIAN A. HOWEY INDIANAPOLIS – Here is the most critical chal- lenge facing President Biden: Vaccinate as many of the 320 million Americans as soon as possible. While the Trump administration’s Operation Warp “Hoosiers have risen to meet Speed helped develop the CO- VID-19 vaccine in record time, these unprecedented challenges. most of the manufactured doses haven’t been injected into the The state of our state is resilient arms of Americans. -

Secretary Alex Azar
April 13, 2020 The Honorable Alex Azar The Honorable Seema Verma Secretary Administrator U.S. Department of Health and Human Services Centers for Medicare and Medicaid Services 200 Independence Ave. SW 7500 Security Blvd. Washington, DC 20201 Baltimore, MD 21244 Dear Secretary Azar and Administrator Verma: In the face of the worst public health crisis in modern history, we as governors are taking unprecedented steps to protect the people of our states from the coronavirus pandemic and the economic devastation occurring in its wake. Many of our states have taken the step of waiving co-payments for coronavirus- related testing and treatment through our Medicaid programs and have encouraged our private insurers to do the same. We all know that more needs to be done to increase access to affordable health care during this crisis, however. To that end, we would ask that you reconsider your decision and immediately open a special enrollment period of at least 30 days on the federal health care exchange. A special enrollment period would ensure individuals in the 38 states on the federal exchange, in addition to those who already qualify, can purchase the coverage they need during this challenging time. Too many of our constituents are uninsured or underinsured despite the steps we’ve taken at the state level. As a result, far too many of our residents are choosing to forgo coronavirus testing and treatment out of fear of the potential costs to themselves and their families at a time of increasing economic distress. Not only is this unacceptable, it’s also dangerous as it undermines our ability as a nation to stop the spread of COVID- 19. -
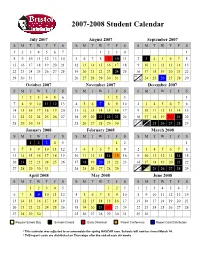
Approved Student Calendar
2007-2008 Student Calendar July 2007 August 2007 September 2007 SMTWT F S SMTWT F S SMTWT F S 1234567 1234 1 8910111213145678910 11 2 3 45678 15 16 17 18 19 20 21 12 13 14 15 16 17 18 9 10 11 12 13 14 15 22 23 24 25 26 27 28 19 20 21 22 23 24 25 16 17 18 19 20 21 22 23 29 30 31 26 27 28 29 30 31 30 24 25 26 27 28 29 October 2007 November 2007 December 2007 SMTWT F S SMTWT F S SMTWT F S 123456 123 1 7891011 12 134567 89102345678 14 15 16 17 18 19 20 11 12 13 14 15 16 17 9 10 11 12 13 14 15 21 22 23 24 25 26 27 18 19 20 21 22 23 24 16 17 18 19 20 21 22 23 24 28 29 30 31 25 26 27 28 29 30 30 31 25 26 27 28 29 January 2008 February 2008 March 2008 SMTWT F S SMTWT F S SMTWT F S 12345 12 1 67891011123456789 2345678 13 14 15 16 17 18 19 10 11 12 13 14 1516 9 1011121314 15 20 21 22 23 24 25 26 17 18 19 20 21 22 23 16 17 18 19 20 21 22 23 24 27 28 29 30 31 24 25 26 27 28 29 30 31 25 26 27 28 29 April 2008 May 2008 June 2008 SMTWT F S SMTWT F S SMTWT F S 12345 123 1234567 6789 10111245678910891011121314 13 14 15 16 17 18 19 11 12 13 14 15 16 17 15 16 17 18 19 20 21 20 21 22 23 24 25 26 18 19 20 21 22 23 24 22 23 24 25 26 27 28 27 28 29 30 25 26 27 28 29 30 31 29 30 Regular School Day Schools Closed Early Dismissal Parent Conference Report Card Distribution * This calendar was adjusted to accommodate the spring NASCAR race. -

Submitted Electronically Via December 23
Submitted electronically via www.regulations.gov December 23, 2020 Alex Azar Secretary Department of Health and Human Services 200 Independence Ave SW Washington, DC 20201 Re: Regulatory Relief to Support Economic Recovery; Request for Information (RFI) Dear Secretary Azar, The Partnership to Advance Virtual Care (Partnership) appreciates the opportunity to respond to the Department of Health and Human Services’ (HHS) request for information (RFI) on the costs and benefits of the regulatory changes beyond the COVID-19 public health emergency (PHE) and which actions should be made permanent. The Partnership is composed of health systems, health IT vendors, innovators, chronic care specialists, and primary care stakeholders who are committed to ensuring patients who receive care via telehealth have access to the highest quality care. As a coalition representing leaders across the telehealth space, we appreciate the opportunity to provide comments on the RFI on Regulatory Relief to Support Economic Recovery. We applaud the broad and swift action taken by HHS and the Centers for Medicare and Medicaid Services (CMS) in response to the pandemic to make certain Medicare beneficiaries continue to have access to necessary care. During the pandemic numerous waivers and flexibilities were issued to ensure access to telehealth services as an alternative modality to in-person care. Prior to the pandemic, telehealth services were limited in scope and utilization. However, out of necessity, the pandemic forced the healthcare system to explore how care can be effectively provided through alternative modalities. The Partnership recognizes that outside of the current PHE, the agency’s authority to eliminate or waive certain telehealth requirements, such as originating and distant site requirements is limited by current statute. -

Summary of Consolidated Financial Statements for the First Quarter of Fiscal Year Ending December 31, 2021 (IFRS)
Summary of Financial Statements for the First Quarter of Fiscal Year Ending December 31, 2021 Summary of Consolidated Financial Statements for the First Quarter of Fiscal Year Ending December 31, 2021 (IFRS) April 30, 2021 Name of listed company: Nabtesco Corporation Stock listed on: First Section of the Tokyo Stock Exchange Code number: 6268 URL: https://www.nabtesco.com Representative: Title: President and CEO Name: Katsuhiro Teramoto Inquiries: Title: General Manager, Corporate Communication Div. Name: Yasushi Minegishi TEL: +81-3-5213-1134 Scheduled date for filing of quarterly report: May 14, 2021 Scheduled dividend payment date: - Quarterly material to supplement the financial results: Yes Quarterly financial results conference: Yes (Teleconference for institutional investors and financial analysts) (Amounts rounded to the nearest million) 1. Consolidated Results for the First Three-month Period of FY2021 (January 1, 2021 to March 31, 2021) (1) Consolidated Operating Results (Percentages indicate year-on-year change) Net income Income Total comprehensive Net sales Operating income Net income attributable to owners before tax income of the parent Million yen % Million yen % Million yen % Million yen % Million yen % Million yen % First Three-month - - - - Period, FY 2021 72,028 5.0 6,655 (18.7) 124,494 81,115 80,058 84,268 First Three-month Period, FY2020 68,616 (2.4) 8,184 33.0 8,162 10.5 5,575 18.5 5,100 24.9 3,551 (33.9) Basic earnings per share Diluted earnings per share Yen Yen First three-month period, FY2021 647.79 647.75 First three-month period, FY2020 41.09 41.08 (2) Consolidated Financial Position Ratio of equity Equity attributable to Total assets Total equity attributable to owners of owners of the parent the parent Million yen Million yen Million yen % As of March 31, 2021 533,840 280,149 267,740 50.2 As of December 31, 2020 351,723 211,641 198,031 56.3 2. -

ACP Letter to Secretary Azar Re: Targeted Provider Relief Fund
May 19, 2020 The Honorable Alex Azar Secretary Department of Health and Human Services 200 Independence Avenue, NW Washington, D.C. 20201 Dear Secretary Azar: On behalf of the American College of Physicians (ACP), I am writing to ask that the Department of Health and Human Services (HHS) make a targeted allocation out of the Provider Relief Fund (PRF) to support primary care physicians and their practices, sufficient to keep their doors open, by offsetting lost revenue from the COVID-19 pandemic, similar to the targeted allocation for rural hospitals. In addition, ACP urges HHS to create more options for primary care practices to transition away from pure fee-for-service (FFS) to per patient per month (PPPM) prospective payments, adjusted for patient characteristics, health status, and risk. These recommendations build upon, and add specificity to, our letter on April 28th where we recommended that a substantial and dedicated portion of the newly authorized $75 billion of the PHSSEF be rapidly and automatically disbursed to physicians and their practices based on lost revenue and increased costs. The American College of Physicians is the largest medical specialty organization and the second- largest physician membership society in the United States. ACP members include 159,000 internal medicine physicians (internists), related subspecialists, and medical students. Internal medicine physicians are specialists who apply scientific knowledge and clinical expertise to the diagnosis, treatment, and compassionate care of adults across the spectrum from health to complex illness. Internal medicine specialists treat many of the patients at greatest risk from COVID-19, including the elderly and patients with pre-existing conditions like diabetes, heart disease, and asthma. -

Daily Unemployment Compensation Data
DISTRICT OF COLUMBIA DOES DISTRICT OF COLUMBIA DEPARTMENT OF DAILY UNEMPLOYMENT EMPLOYMENT SERVICES COMPENSATION DATA Preliminary numbers as of March 4, 2021.* Telephone Date Online Claims Daily Total Running Total Claims March 13, 2020 310 105 415 415 March 14, 2020 213 213 628 March 15, 2020 410 410 1,038 March 16, 2020 1,599 158 1,757 2,795 March 17, 2020 2,541 219 2,760 5,555 March 18, 2020 2,740 187 2,927 8,482 March 19, 2020 2,586 216 2,802 11,284 March 20, 2020 2,726 205 2,931 14,215 March 21, 2020 1,466 1,466 15,681 March 22, 2020 1,240 1,240 16,921 March 23, 2020 2,516 296 2,812 19,733 March 24, 2020 2,156 236 2.392 22,125 March 25, 2020 2,684 176 2,860 24,985 March 26, 2020 2,842 148 2,990 27,975 March 27, 2020 2,642 157 2,799 30,774 March 28, 2020 1,666 25 1,691 32,465 March 29, 2020 1,547 1,547 34,012 March 30, 2020 2,831 186 3,017 37,029 March, 31, 2020 2,878 186 3,064 40,093 April 1, 2020 2,569 186 2,765 42,858 April 2, 2020 2,499 150 2,649 45,507 April 3, 2020 2,071 300 2,371 47,878 April 4, 2020 1,067 14 1,081 48,959 April 5, 2020 1,020 1,020 49,979 April 6, 2020 2,098 155 2,253 52,232 April 7, 2020 1,642 143 1,715 54,017 April 8, 2020 1,486 142 1,628 55,645 *Recalculated and updated daily DISTRICT OF COLUMBIA Telephone DODISTRICT OF CEOLUMBIASDate Online Claims Daily Total Running Total DEPARTMENT OF DAILY UNEMPLOYMENTClaims EMPLOYMENT SERVICES April 9, 2020 1,604 111 1,715 57,360 April 10, 2020 COMPENSATION1,461 119 1,580 DATA58,940 April 11, 2020 763 14 777 59,717 April 12, 2020 698 698 60,415 April 13, 2020 1,499 104 -

Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No
Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No. 33 Class & Market Administrator Payment Dates for Producer Milk Component Final Pool Producer Advance Prices Payment Dates Final Payment Due Partial Payment Due Pool Month Prices Release Date Payrolls Due & Pricing Factors PSF, Admin., MS Cooperative Nonmember Cooperative Nonmember January February 3 * February 13 February 22 December 23, 2020 February 16 ** February 16 February 17 Janaury 25 January 26 February March 3 * March 13 March 22 January 21 * March 15 March 16 March 17 February 25 February 26 March March 31 * April 13 April 22 February 18 * April 15 April 16 April 19 ** March 25 March 26 April May 5 May 13 May 22 March 17 * May 17 ** May 17 ** May 17 April 26 ** April 26 May June 3 * June 13 June 22 April 21 * June 15 June 16 June 17 May 25 May 26 June June 30 * July 13 July 22 May 19 * July 15 July 16 July 19 ** June 25 June 28 ** July August 4 * August 13 August 22 June 23 August 16 ** August 16 August 17 July 26 ** July 26 August September 1 * September 13 September 22 July 21 * September 15 September 16 September 17 August 25 August 26 September September 29 * October 13 October 22 August 18 * October 15 October 18 ** October 18 ** September 27 ** September 27 ** October November 3 * November 13 November 22 September 22 * November 15 November 16 November 17 October 25 October 26 November December 1 * December 13 December 22 October 20 * December 15 December 16 December 17 November 26 ** November 26 December January 5, 2022 January 13, 2022 January 22, 2022 November 17 * January 18, 2022 ** January 18, 2022 ** January 18, 2022 ** December 27 ** December 27 ** * If the release date does not fall on the 5th (Class & Component Prices) or 23rd (Advance Prices & Pricing Factors), the most current release preceding will be used in the price calculation. -
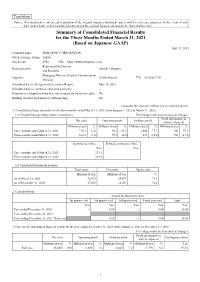
Summary of Consolidated Financial Results for the Three Months Ended
Translation Notice: This document is an excerpt translation of the original Japanese document and is only for reference purposes. In the event of any discrepancy between this translated document and the original Japanese document, the latter shall prevail. Summary of Consolidated Financial Results for the Three Months Ended March 31, 2021 (Based on Japanese GAAP) May 13, 2021 Company name: SEIKO PMC CORPORATION Stock exchange listing: Tokyo Stock code: 4963 URL https://www.seikopmc.co.jp/ Representative Director Representative: Satoshi Takizawa and President Managing Director, Head of Administration Inquiries: Hideki Inouchi TEL 03-6202-7331 Division Scheduled date to file Quarterly Securities Report: May 14, 2021 Scheduled date to commence dividend payments: – Preparation of supplementary material on quarterly financial results: No Holding of quarterly financial results meeting: No (Amounts less than one million yen are rounded down) 1. Consolidated financial results for the three months ended March 31, 2021 (from January 1, 2021 to March 31, 2021) (1) Consolidated operating results (cumulative) Percentages indicate year-on-year changes Profit attributable to Net sales Operating profit Ordinary profit owners of parent Millions of yen % Millions of yen % Millions of yen % Millions of yen % Three months ended March 31, 2021 7,511 13.6 963 62.5 1,054 77.7 700 79.3 Three months ended March 31, 2020 6,613 (1.6) 593 (4.8) 593 (10.6) 390 (21.9) Earnings per share Diluted earnings per share Yen Yen Three months ended March 31, 2021 23.11 – Three months ended March 31, 2020 12.89 – (2) Consolidated financial position Total assets Net assets Equity ratio Millions of yen Millions of yen % As of March 31, 2021 38,972 29,477 71.1 As of December 31, 2020 37,069 28,451 72.4 2. -

AGENDA March 30, 2021 9:00 AM Chambers - Basement Level 1010 10Th Street Modesto, CA 95354
BOARD OF SUPERVISORS Buck Condit, District 1 Vito Chiesa, District 2 Terry Withrow, District 3 Mani Grewal, District 4 Channce Condit, District 5 1010 10th Street Modesto, CA 95354 Phone: 209-525-4494 Fax 209-525-4420 AGENDA March 30, 2021 9:00 AM Chambers - Basement Level 1010 10th Street Modesto, CA 95354 www.stancounty.com/board/index.shtm Public Access to the Board of Supervisors Meetings Members of the public may observe the meeting and provide comments to the Board as described below. • This meeting will be open to the public. Effective June 22, 2020, pursuant to the order issued by Governor Newsom and consistent with guidance issued by the California Department of Public Health, social distancing and face coverings are required for in person attendance at the meeting. The chamber’s audience seating capacity will be limited to approximately thirty (30) persons. • You can also observe the live stream of the meeting at http://www.stancounty.com/sclive/. • In addition, Board meetings are broadcast live and replayed on local cable television. A list of cable channels and broadcast times are available at the following website: www.stancounty.com/board/broadcasting-schedule.shtm • If you would like to provide a written comment, submit your comments via email by 4:00 p.m. on Monday, the day before the meeting. Please email your comment to the Clerk of the Board at [email protected] and include the Agenda Item Number or Public Comment Period in the subject line of the email. The Clerk may read written comments into the record (limited to 250 words or less), if specifically requested to do so at the beginning of your email. -

Early Dance Division Calendar 17-18
Early Dance Division 2017-2018 Session 1 September 9 – November 3 Monday Classes Tuesday Classes September 11 Class September 12 Class September 18 Class September 19 Class September 25 Class September 26 Class October 2 Class October 3 Class October 9 Class October 10 Class October 16 Class October 17 Class October 23 Class October 24 Class October 30 Last Class October 31 Last Class Wednesday Classes Thursday Classes September 13 Class September 14 Class September 20 Class September 21* Class September 27 Class September 28 Class October 4 Class October 5 Class October 11 Class October 12 Class October 18 Class October 19 Class October 25 Class October 26 Class November 1 Last Class November 2 Last Class Saturday Classes Sunday Classes September 9 Class September 10 Class September 16 Class September 17 Class September 23 Class September 24 Class September 30* Class October 1 Class October 7 Class October 8 Class October 14 Class October 15 Class October 21 Class October 22 Class October 28 Last Class October 29 Last Class *Absences due to the holiday will be granted an additional make-up class. Early Dance Division 2017-2018 Session 2 November 4 – January 22 Monday Classes Tuesday Classes November 6 Class November 7 Class November 13 Class November 14 Class November 20 No Class November 21 No Class November 27 Class November 28 Class December 4 Class December 5 Class December 11 Class December 12 Class December 18 Class December 19 Class December 25 No Class December 26 No Class January 1 No Class January 2 No Class January 8 Class -
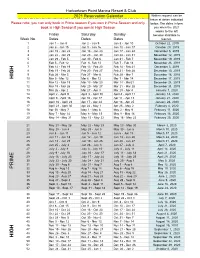
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May