Appendix Glossary of Terms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -
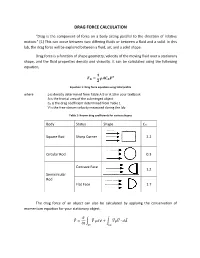
Drag Force Calculation
DRAG FORCE CALCULATION “Drag is the component of force on a body acting parallel to the direction of relative motion.” [1] This can occur between two differing fluids or between a fluid and a solid. In this lab, the drag force will be explored between a fluid, air, and a solid shape. Drag force is a function of shape geometry, velocity of the moving fluid over a stationary shape, and the fluid properties density and viscosity. It can be calculated using the following equation, ퟏ 푭 = 흆푨푪 푽ퟐ 푫 ퟐ 푫 Equation 1: Drag force equation using total profile where ρ is density determined from Table A.9 or A.10 in your textbook A is the frontal area of the submerged object CD is the drag coefficient determined from Table 1 V is the free-stream velocity measured during the lab Table 1: Known drag coefficients for various shapes Body Status Shape CD Square Rod Sharp Corner 2.2 Circular Rod 0.3 Concave Face 1.2 Semicircular Rod Flat Face 1.7 The drag force of an object can also be calculated by applying the conservation of momentum equation for your stationary object. 휕 퐹⃗ = ∫ 푉⃗⃗ 휌푑∀ + ∫ 푉⃗⃗휌푉⃗⃗ ∙ 푑퐴⃗ 휕푡 퐶푉 퐶푆 Assuming steady flow, the equation reduces to 퐹⃗ = ∫ 푉⃗⃗휌푉⃗⃗ ∙ 푑퐴⃗ 퐶푆 The following frontal view of the duct is shown below. Integrating the velocity profile after the shape will allow calculation of drag force per unit span. Figure 1: Velocity profile after an inserted shape. Combining the previous equation with Figure 1, the following equation is obtained: 푊 퐷푓 = ∫ 휌푈푖(푈∞ − 푈푖)퐿푑푦 0 Simplifying the equation, you get: 20 퐷푓 = 휌퐿 ∑ 푈푖(푈∞ − 푈푖)훥푦 푖=1 Equation 2: Drag force equation using wake profile The pressure measurements can be converted into velocity using the Bernoulli’s equation as follows: 2Δ푃푖 푈푖 = √ 휌퐴푖푟 Be sure to remember that the manometers used are in W.C. -

Chapter 4: Immersed Body Flow [Pp
MECH 3492 Fluid Mechanics and Applications Univ. of Manitoba Fall Term, 2017 Chapter 4: Immersed Body Flow [pp. 445-459 (8e), or 374-386 (9e)] Dr. Bing-Chen Wang Dept. of Mechanical Engineering Univ. of Manitoba, Winnipeg, MB, R3T 5V6 When a viscous fluid flow passes a solid body (fully-immersed in the fluid), the body experiences a net force, F, which can be decomposed into two components: a drag force F , which is parallel to the flow direction, and • D a lift force F , which is perpendicular to the flow direction. • L The drag coefficient CD and lift coefficient CL are defined as follows: FD FL CD = 1 2 and CL = 1 2 , (112) 2 ρU A 2 ρU Ap respectively. Here, U is the free-stream velocity, A is the “wetted area” (total surface area in contact with fluid), and Ap is the “planform area” (maximum projected area of an object such as a wing). In the remainder of this section, we focus our attention on the drag forces. As discussed previously, there are two types of drag forces acting on a solid body immersed in a viscous flow: friction drag (also called “viscous drag”), due to the wall friction shear stress exerted on the • surface of a solid body; pressure drag (also called “form drag”), due to the difference in the pressure exerted on the front • and rear surfaces of a solid body. The friction drag and pressure drag on a finite immersed body are defined as FD,vis = τwdA and FD, pres = pdA , (113) ZA ZA Streamwise component respectively. -

A New Occurrence of Terrestrial Native Iron in the Earth's Surface
geosciences Article A New Occurrence of Terrestrial Native Iron in the Earth’s Surface: The Ilia Thermogenic Travertine Case, Northwestern Euboea, Greece Christos Kanellopoulos 1,2,* ID , Eugenia Valsami-Jones 3,4, Panagiotis Voudouris 1, Christina Stouraiti 1 ID , Robert Moritz 2, Constantinos Mavrogonatos 1 ID and Panagiotis Mitropoulos 1,† 1 Department of Geology and Geoenvironment, National and Kapodistrian University of Athens, Panepistimioupolis Zografou, 15784 Athens, Greece; [email protected] (P.V.); [email protected] (C.S.); [email protected] (C.M.); [email protected] (P.M.) 2 Section of Earth and Environmental Sciences, University of Geneva, Rue des Maraichers 13, 1205 Geneva, Switzerland; [email protected] 3 School of Geography, Earth & Environmental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK; [email protected] 4 Department of Earth Sciences, Natural History Museum London, Cromwell Road, London SW7 5BD, UK * Correspondence: [email protected] † Professor Panagiotis Mitropoulos has passed away in 2017. Received: 6 April 2018; Accepted: 23 July 2018; Published: 31 July 2018 Abstract: Native iron has been identified in an active thermogenic travertine deposit, located at Ilia area (Euboea Island, Greece). The deposit is forming around a hot spring, which is part of a large active metallogenetic hydrothermal system depositing ore-bearing travertines. The native iron occurs in two shapes: nodules with diameter 0.4 and 0.45 cm, and angular grains with length up to tens of µm. The travertine laminae around the spherical/ovoid nodules grow smoothly, and the angular grains are trapped inside the pores of the travertine. -

Stoichiometry: the Reaction of Iron with Copper(II) Sulfate
CEAC 103 GENERAL CHEMISTRY Experiment 2 Stoichiometry: The Reaction of Iron with Copper(II) Sulfate Purpose: To enhance the understanding of stoichiometry, a reaction between iron and copper (II) sulfate solution will be conducted. This will help you to differentiate limiting and excess reactant in a chemical reaction. Finally the theoretical and percent yield of this reaction will be calculated. Theory Stoichiometry is the measurement of quantitative relationships in chemical formulas and equations. In this experiment stoichiometric principles will be used to obtain the appropriate equation between the reaction of iron metal and copper(II) sulfate solution. After the reaction is taking place, the formation of metallic copper, which is seen precipitating as a finely divided reddish-orange powder will be observed. This reaction is one of the example of single substitution reaction in which one element “displaces” from a compound by another element. The element which has ability of displacing other element from compound is said to be “more active” than the displaced metal. In this experiment, iron is more active than copper. Two distinct forms of iron are present, namely Fe2+ and Fe3+. Stoichiometric principles will be used to determine which reaction is more dominant compared to other one by examining the reaction between iron and copper (II) sulfate solution. If Fe2+ is formed, then equation (1) is dominant, while equation (2) will be selected if Fe3+ is formed. This can be determined 1 according to mole ratio of copper to iron. If the moles of copper is equal to the moles of iron, then equation (1) has taken place. -

UNIT – 4 FORCES on IMMERSED BODIES Lecture-01
1 UNIT – 4 FORCES ON IMMERSED BODIES Lecture-01 Forces on immersed bodies When a body is immersed in a real fluid, which is flowing at a uniform velocity U, the fluid will exert a force on the body. The total force (FR) can be resolved in two components: 1. Drag (FD): Component of the total force in the direction of motion of fluid. 2. Lift (FL): Component of the total force in the perpendicular direction of the motion of fluid. It occurs only when the axis of the body is inclined to the direction of fluid flow. If the axis of the body is parallel to the fluid flow, lift force will be zero. Expression for Drag & Lift Forces acting on the small elemental area dA are: i. Pressure force acting perpendicular to the surface i.e. p dA ii. Shear force acting along the tangential direction to the surface i.e. τ0dA (a) Drag force (FD) : Drag force on elemental area = p dAcosθ + τ0 dAcos(90 – θ = p dAosθ + τ0dAsinθ Hence Total drag (or profile drag) is given by, Where �� = ∫ � cos � �� + ∫�0 sin � �� = pressure drag or form drag, and ∫ � cos � �� = shear drag or friction drag or skin drag (b) Lift0 force (F ) : ∫ � sin � ��L Lift force on the elemental area = − p dAsinθ + τ0 dA sin(90 – θ = − p dAsiθ + τ0dAcosθ Hence, total lift is given by http://www.rgpvonline.com �� = ∫�0 cos � �� − ∫ p sin � �� 2 The drag & lift for a body moving in a fluid of density at a uniform velocity U are calculated mathematically as 2 � And �� = � � � 2 � Where A = projected area of the body or�� largest= � project� � area of the immersed body. -

Iron –Carbon Phase Diagram
IRON –CARBON PHASE DIAGRAM CB.EN.P2MFG15018 Definition of structures: Various phases that appear on the Iron- Carbon equilibrium phase diagram are as under: • Austenite • Ferrite • Pearlite • Cementite • Martensite • Ledeburite Definition of structures: Austenite is an interstitial solid solution of Carbon dissolved in (F.C.C.) iron. Maximum solubility is 2.0 % C at 1130°C. High formability, most of heat treatments begin with this single phase. It is normally not stable at room temperature. But, under certain conditions it is possible to obtain austenite at room temperature. Austenite Average properties are: Tensile strength = 150,000 psi; Elongation = 10 percent in 2 in.; Hardness = Rockwell C 40, approx; and toughness = high Definition of structures: Ferrite is known as α solid solution. It is an interstitial solid solution of a small amount of carbon dissolved in α (BCC) iron. stable form of iron below 912 deg.C. The maximum solubility is 0.025 % C at 723C and it dissolves only 0.008 % C at room temperature. It is the softest structure that appears on the diagram. Ferrite Average properties are: Tensile strength = 40,000 psi; Elongation = 40 % in 2 in; Hardness > Rockwell C 0 or > Rockwell B 90 Definition of structures: Pearlite is the eutectoid mixture containing 0.80 % C and is formed at 723°C on very slow cooling. It is a very fine platelike or lamellar mixture of ferrite and cementite. The white ferritic background or matrix contains thin plates of cementite (dark). Pearlite Average properties are: Tensile strength = 120,000 psi; Elongation = 20 % in 2 in.; Hardness = Rockwell C20, BHN-300 Definition of structures: Cementite or iron carbide, is very hard, brittle intermetallic compound of iron & carbon, as Fe3C, contains 6.67 % C. -

Copper Alloys
THE COPPER ADVANTAGE A Guide to Working With Copper and Copper Alloys www.antimicrobialcopper.com CONTENTS I. Introduction ............................. 3 PREFACE Conductivity .....................................4 Strength ..........................................4 The information in this guide includes an overview of the well- Formability ......................................4 known physical, mechanical and chemical properties of copper, Joining ...........................................4 as well as more recent scientific findings that show copper has Corrosion ........................................4 an intrinsic antimicrobial property. Working and finishing Copper is Antimicrobial ....................... 4 techniques, alloy families, coloration and other attributes are addressed, illustrating that copper and its alloys are so Color ..............................................5 adaptable that they can be used in a multitude of applications Copper Alloy Families .......................... 5 in almost every industry, from door handles to electrical circuitry to heat exchangers. II. Physical Properties ..................... 8 Copper’s malleability, machinability and conductivity have Properties ....................................... 8 made it a longtime favorite metal of manufacturers and Electrical & Thermal Conductivity ........... 8 engineers, but it is its antimicrobial property that will extend that popularity into the future. This guide describes that property and illustrates how it can benefit everything from III. Mechanical -

Chapter 4: Immersed Body Flow [Pp
MECH 3492 Fluid Mechanics and Applications Univ. of Manitoba Fall Term, 2017 Chapter 4: Immersed Body Flow [pp. 445-459 (8e), or 374-386 (9e)] Dr. Bing-Chen Wang Dept. of Mechanical Engineering Univ. of Manitoba, Winnipeg, MB, R3T 5V6 When a viscous fluid flow passes a solid body (fully-immersed in the fluid), the body experiences a net force, F, which can be decomposed into two components: a drag force F , which is parallel to the flow direction, and • D a lift force F , which is perpendicular to the flow direction. • L The drag coefficient CD and lift coefficient CL are defined as follows: FD FL CD = 1 2 and CL = 1 2 , (112) 2 ρU A 2 ρU Ap respectively. Here, U is the free-stream velocity, A is the “wetted area” (total surface area in contact with fluid), and Ap is the “planform area” (maximum projected area of an object such as a wing). In the remainder of this section, we focus our attention on the drag forces. As discussed previously, there are two types of drag forces acting on a solid body immersed in a viscous flow: friction drag (also called “viscous drag”), due to the wall friction shear stress exerted on the • surface of a solid body; pressure drag (also called “form drag”), due to the difference in the pressure exerted on the front • and rear surfaces of a solid body. The friction drag and pressure drag on a finite immersed body are defined as FD,vis = τwdA and FD, pres = pdA , (113) ZA ZA Streamwise component respectively. -

Hydraulics Manual Glossary G - 3
Glossary G - 1 GLOSSARY OF HIGHWAY-RELATED DRAINAGE TERMS (Reprinted from the 1999 edition of the American Association of State Highway and Transportation Officials Model Drainage Manual) G.1 Introduction This Glossary is divided into three parts: · Introduction, · Glossary, and · References. It is not intended that all the terms in this Glossary be rigorously accurate or complete. Realistically, this is impossible. Depending on the circumstance, a particular term may have several meanings; this can never change. The primary purpose of this Glossary is to define the terms found in the Highway Drainage Guidelines and Model Drainage Manual in a manner that makes them easier to interpret and understand. A lesser purpose is to provide a compendium of terms that will be useful for both the novice as well as the more experienced hydraulics engineer. This Glossary may also help those who are unfamiliar with highway drainage design to become more understanding and appreciative of this complex science as well as facilitate communication between the highway hydraulics engineer and others. Where readily available, the source of a definition has been referenced. For clarity or format purposes, cited definitions may have some additional verbiage contained in double brackets [ ]. Conversely, three “dots” (...) are used to indicate where some parts of a cited definition were eliminated. Also, as might be expected, different sources were found to use different hyphenation and terminology practices for the same words. Insignificant changes in this regard were made to some cited references and elsewhere to gain uniformity for the terms contained in this Glossary: as an example, “groundwater” vice “ground-water” or “ground water,” and “cross section area” vice “cross-sectional area.” Cited definitions were taken primarily from two sources: W.B. -

Alloys: Making an Alloy
Inspirational chemistry 21 Alloys: making an alloy Index 2.3.1 2 sheets In this experiment, students make an alloy (solder) from tin and lead and compare its properties to those of pure lead. Equipment required Per pair or group of students: ■ About 2 g lead ■ About 2 g tin ■ Crucible ■ Pipe clay triangle ■ Bunsen, tripod and heatproof mat ■ Spatula ■ Carbon powder – 1 spatula per student ■ Tongs ■ 2 sand trays or sturdy metal lids ■ Sand ■ Access to a balance ■ Eye protection. Health and safety The most likely incident in this experiment is a student burning themselves so warn them that the equipment will be hot. Pouring molten metal can be hazardous if you are not sure how to use tongs correctly – it would be worth demonstrating how to use them safely. Some tongs in schools do not grip well. Every pair must be checked before the start of the experiment. Eye protection should be worn. Lead is a toxic metal. If it is heated for too long or too high above its melting point it could start to give off fumes. Ensure that the laboratory is well ventilated, warn students against breathing in the fumes given off by their sample during the experiment and tell them to heat the metals no longer than is necessary to get them to melt. 22 Inspirational chemistry Results Hardness testing should show clearly that the alloy is harder than the pure lead. The alloy can be used to scratch the lead convincingly. The lead does not leave a mark on the alloy. (Students may need to be reminded how to do this simple test – just try to scratch one metal with the other.) The density of the alloy should be less than that of the lead, but this test is fairly subjective. -

Why We Need to Assure Adequacy of Zinc
Aunt Cathy’s Guide to Nutrition: Sanford Nutrition Therapy Dept. 1/15 Thinking about Clinical/Medical Nutrition Issues and Applications of RDAs, RDI, DRIs, ULS, AIs, etc.: Why We Need to Assure Adequacy of Zinc (from the series “How Am I Supposed to Remember All This Stuff?!”) Aunt Cathy Cathy Breedon PhD, RD, CSP, FADA, FAND Clinical and Metabolic Nutrition Specialist / Prenatal/Pediatric Nutrition Specialist Sanford Medical Center, and Clinical Associate Professor UND School of Medicine, Fargo, ND • Zinc is a necessary mineral cofactor needed for over 200 enzymes to work. • Not having enough screws stuff up a LOT. • What kinds of things won’t work well if zinc is inadequate? Read on … 1 Memory Tricks for ZINC Many people remember things best if they also see pictures, so here are some pictures identifying some specific places in the body for which zinc is absolutely essential. Other folks remember things best if they hear them … so if you are one of those people, be sure to read the descriptor for each function of zinc out loud. The Zinc Story: “Zinco” (Zorro’s more irritating cousin) has just marked the places where people need zinc for their bodies to operate. Just picture the fellow on the left to remember that zinc is needed all over the body. It is a co-factor for over 200 enzymes! In other words … I dare you to name a body part that is not dependent on adequacy of zinc! Here are some specific places where he marked things with a Z for Zinc: Zinc is need to make DNA So Zinc is needed And Zinc is needed to to make new cells for babies