Supplementary Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

KRBA1 CRISPR/Cas9 KO Plasmid (M): Sc-429589
SANTA CRUZ BIOTECHNOLOGY, INC. KRBA1 CRISPR/Cas9 KO Plasmid (m): sc-429589 BACKGROUND APPLICATIONS The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and KRBA1 CRISPR/Cas9 KO Plasmid (m) is recommended for the disruption of CRISPR-associated protein (Cas9) system is an adaptive immune response gene expression in mouse cells. defense mechanism used by archea and bacteria for the degradation of for- eign genetic material (4,6). This mechanism can be repurposed for other 20 nt non-coding RNA sequence: guides Cas9 functions, including genomic engineering for mammalian systems, such as to a specific target location in the genomic DNA gene knockout (KO) (1,2,3,5). CRISPR/Cas9 KO Plasmid products enable the U6 promoter: drives gRNA scaffold: helps Cas9 identification and cleavage of specific genes by utilizing guide RNA (gRNA) expression of gRNA bind to target DNA sequences derived from the Genome-scale CRISPR Knock-Out (GeCKO) v2 library developed in the Zhang Laboratory at the Broad Institute (3,5). Termination signal Green Fluorescent Protein: to visually REFERENCES verify transfection CRISPR/Cas9 Knockout Plasmid CBh (chicken β-Actin 1. Cong, L., et al. 2013. Multiplex genome engineering using CRISPR/Cas hybrid) promoter: drives systems. Science 339: 819-823. 2A peptide: expression of Cas9 allows production of both Cas9 and GFP from the 2. Mali, P., et al. 2013. RNA-guided human genome engineering via Cas9. same CBh promoter Science 339: 823-826. Nuclear localization signal 3. Ran, F.A., et al. 2013. Genome engineering using the CRISPR-Cas9 system. Nuclear localization signal SpCas9 ribonuclease Nat. Protoc. 8: 2281-2308. -

Screening and Identification of Key Biomarkers in Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Screening and identification of key biomarkers in clear cell renal cell carcinoma based on bioinformatics analysis Basavaraj Vastrad1, Chanabasayya Vastrad*2 , Iranna Kotturshetti 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. 3. Department of Ayurveda, Rajiv Gandhi Education Society`s Ayurvedic Medical College, Ron, Karnataka 562209, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Clear cell renal cell carcinoma (ccRCC) is one of the most common types of malignancy of the urinary system. The pathogenesis and effective diagnosis of ccRCC have become popular topics for research in the previous decade. In the current study, an integrated bioinformatics analysis was performed to identify core genes associated in ccRCC. An expression dataset (GSE105261) was downloaded from the Gene Expression Omnibus database, and included 26 ccRCC and 9 normal kideny samples. Assessment of the microarray dataset led to the recognition of differentially expressed genes (DEGs), which was subsequently used for pathway and gene ontology (GO) enrichment analysis. -

Topological Scoring of Protein Interaction Networks
bioRxiv preprint doi: https://doi.org/10.1101/438408; this version posted October 8, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Topological Scoring of Protein Interaction Networks Mihaela E. Sardiu1, Joshua M. Gilmore1,2, Brad D. Groppe1,3, Arnob Dutta1,4, Laurence Florens1, and Michael P. Washburn1,5‡ 1Stowers Institute for Medical Research, Kansas City, MO 64110 U.S.A. 2Current Address: Boehringer Ingelheim Vetmedica, St. Joseph, MO 64506 U.S.A. 3Current Address: Thermo Fisher Scientific, Waltham, MA 02451, U.S.A. 4Current Address: Department of Cell and Molecular Biology, University of Rhode Island, 287 CBLS, 120 Flagg Road, Kingston, RI 02881. 5 Department of Pathology and Laboratory Medicine, The University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, Kansas 66160, USA ‡To whom correspondence should be addressed: Michael Washburn, Ph.D. Stowers Institute for Medical Research 1000 E. 50th St. Kansas City, MO 64110 Phone: 816-926-4457 E-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/438408; this version posted October 8, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract It remains a significant challenge to define individual protein associations within networks where an individual protein can directly interact with other proteins and/or be part of large complexes, which contain functional modules. Here we demonstrate the topological scoring (TopS) algorithm for the analysis of quantitative proteomic analyses of affinity purifications. -

Reprogramming of Trna Modifications Controls the Oxidative Stress Response by Codon-Biased Translation of Proteins
Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Chan, Clement T.Y. et al. “Reprogramming of tRNA Modifications Controls the Oxidative Stress Response by Codon-biased Translation of Proteins.” Nature Communications 3 (2012): 937. As Published http://dx.doi.org/10.1038/ncomms1938 Publisher Nature Publishing Group Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/76775 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins Clement T.Y. Chan,1,2 Yan Ling Joy Pang,1 Wenjun Deng,1 I. Ramesh Babu,1 Madhu Dyavaiah,3 Thomas J. Begley3 and Peter C. Dedon1,4* 1Department of Biological Engineering, 2Department of Chemistry and 4Center for Environmental Health Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139; 3College of Nanoscale Science and Engineering, University at Albany, SUNY, Albany, NY 12203 * Corresponding author: PCD, Department of Biological Engineering, NE47-277, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139; tel 617-253-8017; fax 617-324-7554; email [email protected] 2 ABSTRACT Selective translation of survival proteins is an important facet of cellular stress response. We recently demonstrated that this translational control involves a stress-specific reprogramming of modified ribonucleosides in tRNA. -

Allele-Specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish
Allele-specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish by Ailu Chen A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama August 1, 2015 Keywords: catfish, interspecific hybrids, allele-specific expression, ribosomal protein Copyright 2015 by Ailu Chen Approved by Zhanjiang Liu, Chair, Professor, School of Fisheries, Aquaculture and Aquatic Sciences Nannan Liu, Professor, Entomology and Plant Pathology Eric Peatman, Associate Professor, School of Fisheries, Aquaculture and Aquatic Sciences Aaron M. Rashotte, Associate Professor, Biological Sciences Abstract Interspecific hybridization results in a vast reservoir of allelic variations, which may potentially contribute to phenotypical enhancement in the hybrids. Whether the allelic variations are related to the downstream phenotypic differences of interspecific hybrid is still an open question. The recently developed genome-wide allele-specific approaches that harness high- throughput sequencing technology allow direct quantification of allelic variations and gene expression patterns. In this work, I investigated allele-specific expression (ASE) pattern using RNA-Seq datasets generated from interspecific catfish hybrids. The objective of the study is to determine the ASE genes and pathways in which they are involved. Specifically, my study investigated ASE-SNPs, ASE-genes, parent-of-origins of ASE allele and how ASE would possibly contribute to heterosis. My data showed that ASE was operating in the interspecific catfish system. Of the 66,251 and 177,841 SNPs identified from the datasets of the liver and gill, 5,420 (8.2%) and 13,390 (7.5%) SNPs were identified as significant ASE-SNPs, respectively. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Wnt/Β-Catenin Signaling Induces MLL to Create Epigenetic Changes In
Wend et al. page 1 Wnt/ȕ-catenin signaling induces MLL to create epigenetic changes in salivary gland tumors Peter Wend1,7, Liang Fang1, Qionghua Zhu1, Jörg H. Schipper2, Christoph Loddenkemper3, Frauke Kosel1, Volker Brinkmann4, Klaus Eckert1, Simone Hindersin2, Jane D. Holland1, Stephan Lehr5, Michael Kahn6, Ulrike Ziebold1,*, Walter Birchmeier1,* 1Max-Delbrueck Center for Molecular Medicine, Robert-Rössle-Str. 10, 13125 Berlin, Germany 2Department of Otorhinolaryngology, University Hospital Düsseldorf, Moorenstr. 5, 40225 Düsseldorf, Germany 3Institute of Pathology, Charité-UKBF, Hindenburgdamm 30, 12200 Berlin, Germany 4Max Planck Institute for Infection Biology, Schumannstr. 20, 10117 Berlin, Germany 5Baxter Innovations GmbH, Wagramer Str. 17-19, 1220 Vienna, Austria 6Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research, University of Southern California, Los Angeles, CA 90033, USA 7Present address: David Geffen School of Medicine and Jonsson Comprehensive Cancer Center, University of California at Los Angeles, CA 90095, USA *These senior authors contributed equally to this work. Contact: [email protected], phone: +49-30-9406-3800, fax: +49-30-9406-2656 Running title: ȕ-catenin and MLL drive salivary gland tumors Characters (including spaces): 52,604 Wend et al. page 2 Abstract We show that activation of Wnt/ȕ-catenin and attenuation of Bmp signals, by combined gain- and loss-of-function mutations of ȕ-catenin and Bmpr1a, respectively, results in rapidly growing, aggressive squamous cell carcinomas (SCC) in the salivary glands of mice. Tumors contain transplantable and hyper-proliferative tumor propagating cells, which can be enriched by FACS. Single mutations stimulate stem cells, but tumors are not formed. We show that ȕ-catenin, CBP and Mll promote self- renewal and H3K4 tri-methylation in tumor propagating cells. -

Supp Material.Pdf
Simon et al. Supplementary information: Table of contents p.1 Supplementary material and methods p.2-4 • PoIy(I)-poly(C) Treatment • Flow Cytometry and Immunohistochemistry • Western Blotting • Quantitative RT-PCR • Fluorescence In Situ Hybridization • RNA-Seq • Exome capture • Sequencing Supplementary Figures and Tables Suppl. items Description pages Figure 1 Inactivation of Ezh2 affects normal thymocyte development 5 Figure 2 Ezh2 mouse leukemias express cell surface T cell receptor 6 Figure 3 Expression of EZH2 and Hox genes in T-ALL 7 Figure 4 Additional mutation et deletion of chromatin modifiers in T-ALL 8 Figure 5 PRC2 expression and activity in human lymphoproliferative disease 9 Figure 6 PRC2 regulatory network (String analysis) 10 Table 1 Primers and probes for detection of PRC2 genes 11 Table 2 Patient and T-ALL characteristics 12 Table 3 Statistics of RNA and DNA sequencing 13 Table 4 Mutations found in human T-ALLs (see Fig. 3D and Suppl. Fig. 4) 14 Table 5 SNP populations in analyzed human T-ALL samples 15 Table 6 List of altered genes in T-ALL for DAVID analysis 20 Table 7 List of David functional clusters 31 Table 8 List of acquired SNP tested in normal non leukemic DNA 32 1 Simon et al. Supplementary Material and Methods PoIy(I)-poly(C) Treatment. pIpC (GE Healthcare Lifesciences) was dissolved in endotoxin-free D-PBS (Gibco) at a concentration of 2 mg/ml. Mice received four consecutive injections of 150 μg pIpC every other day. The day of the last pIpC injection was designated as day 0 of experiment. -

PRODUCT SPECIFICATION Prest Antigen KRBA1 Product Datasheet
PrEST Antigen KRBA1 Product Datasheet PrEST Antigen PRODUCT SPECIFICATION Product Name PrEST Antigen KRBA1 Product Number APrEST89619 Gene Description KRAB-A domain containing 1 Alternative Gene KIAA1862 Names Corresponding Anti-KRBA1 (HPA050448) Antibodies Description Recombinant protein fragment of Human KRBA1 Amino Acid Sequence Recombinant Protein Epitope Signature Tag (PrEST) antigen sequence: DLQGLSRGTARRARPLPPDAPPAEPPGLHCSSSQQLLSSTPSCHAAPPAH PLLAHTGGHQSPLPPLVPAALPLQGASPPAASADADVPTSGVAPDGIPER PKEPSSLLGGVQRALQEELWGGEHRDPRW Fusion Tag N-terminal His6ABP (ABP = Albumin Binding Protein derived from Streptococcal Protein G) Expression Host E. coli Purification IMAC purification Predicted MW 31 kDa including tags Usage Suitable as control in WB and preadsorption assays using indicated corresponding antibodies. Purity >80% by SDS-PAGE and Coomassie blue staining Buffer PBS and 1M Urea, pH 7.4. Unit Size 100 µl Concentration Lot dependent Storage Upon delivery store at -20°C. Avoid repeated freeze/thaw cycles. Notes Gently mix before use. Optimal concentrations and conditions for each application should be determined by the user. Product of Sweden. For research use only. Not intended for pharmaceutical development, diagnostic, therapeutic or any in vivo use. No products from Atlas Antibodies may be resold, modified for resale or used to manufacture commercial products without prior written approval from Atlas Antibodies AB. Warranty: The products supplied by Atlas Antibodies are warranted to meet stated product specifications and to conform to label descriptions when used and stored properly. Unless otherwise stated, this warranty is limited to one year from date of sales for products used, handled and stored according to Atlas Antibodies AB's instructions. Atlas Antibodies AB's sole liability is limited to replacement of the product or refund of the purchase price. -

Targeted Deletion of the Murine Corneodesmosin Gene Delineates Its Essential Role in Skin and Hair Physiology
Targeted deletion of the murine corneodesmosin gene delineates its essential role in skin and hair physiology Mitsuru Matsumotoa,b, Yiqing Zhouc, Shinji Matsuod, Hideki Nakanishid, Kenji Hirosee, Hajimu Ourae, Seiji Arasee, Akemi Ishida-Yamamotof, Yoshimi Bandog, Keisuke Izumig, Hiroshi Kiyonarih, Naoko Oshimah, Rika Nakayamah, Akemi Matsushimaa, Fumiko Hirotaa, Yasuhiro Mouria, Noriyuki Kurodaa, Shigetoshi Sanoi, and David D. Chaplinj aDivision of Molecular Immunology, Institute for Enzyme Research, University of Tokushima, Tokushima 770-8503, Japan; cKortex Laboratories, Orange Village, OH 44022-1412; dSection of Plastic and Reconstructive Surgery, University Hospital, University of Tokushima, Tokushima 770-8503, Japan; eDepartment of Dermatology, Institute of Health Biosciences, University of Tokushima Graduate School, Tokushima 770-8503, Japan; fDepartment of Dermatology, Asahikawa Medical College, Asahikawa 078-8510, Japan; gDepartment of Molecular and Environmental Pathology, Institute of Health Biosciences, University of Tokushima Graduate School, Tokushima 770-8503, Japan; hLaboratory for Animal Resources and Genetic Engineering, Center for Developmental Biology, RIKEN Kobe, Kobe 650-0047, Japan; iDepartment of Dermatology, Kochi University School of Medicine, Nankoku 783-8505, Japan; and jDepartment of Microbiology, University of Alabama at Birmingham, Birmingham, AL 35294-2170 Edited by Kathryn V. Anderson, Sloan–Kettering Institute, New York, NY, and approved February 29, 2008 (received for review October 1, 2007) Controlled proteolytic degradation of specialized junctional struc- gene are associated with susceptibility to psoriasis (10–12), a tures, corneodesmosomes, by epidermal proteases is an essential chronic inflammatory disorder of the skin characterized by process for physiological desquamation of the skin. Corneodesmo- excessive growth and aberrant differentiation of keratinocytes sin (CDSN) is an extracellular component of corneodesmosomes (13). -

Epidermal Detachment, Desmosomal Dissociation, and Destabilization of Corneodesmosin in Spink5-/- Mice
Downloaded from genesdev.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION conserved cysteines. Domains 2 and 15 possess two ad- Epidermal detachment, ditional cysteines, which make them typical Kazal-type desmosomal dissociation, proteinase inhibitor domains (Magert et al. 1999). LEKTI exhibits proteinase inhibitor activity in vitro (Magert et and destabilization al. 1999; Komatsu et al. 2002; Walden et al. 2002; Mit- of corneodesmosin sudo et al. 2003). In NS patients, loss or reduction of −/− LEKTI activity is presumed to result in elevated proteo- in Spink5 mice lytic activity in the suprabasal epidermis, leading to erythroderma and skin-barrier defects. However, the spe- Tao Yang,1,2,6 Dongcai Liang,1 Peter J. Koch,1,3 cific proteins that are targeted for degradation in these Daniel Hohl,5 Farrah Kheradmand,4 and patients have not been identified. We describe here a Paul A. Overbeek1,2,7 Spink5 mutant mouse line that shows severe skin de- fects associated with desmosomal fragility, and thus, 1Department of Molecular and Cellular Biology, 2Department provides insights into the molecular pathogenesis of NS of Molecular and Human Genetics, 3Department of and a novel model system for studies of keratinocyte Dermatology, 4Biology of Inflammation Center and adhesion. Department of Medicine, Baylor College of Medicine, Houston, Texas 77030, USA; 5Laboratory for Cutaneous Results and Discussion Biology, Dermatology Unit, Beaumont Hospital, CHUV, Transgenic mouse line OVE1498 was generated by coin- Lausanne CH-1011, Switzerland jection of a tyrosinase-tagged (Yokoyama et al. 1990) Netherton syndrome (NS) is a human autosomal reces- Sleeping Beauty transposon (Ivics et al. -
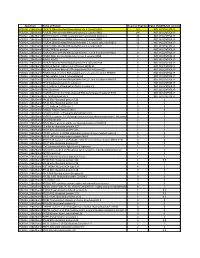
Attachment PDF Icon
Spectrum Name of Protein Count of Peptides Ratio (POL2RA/IgG control) POLR2A_228kdBand POLR2A DNA-directed RNA polymerase II subunit RPB1 197 NOT IN CONTROL IP POLR2A_228kdBand POLR2B DNA-directed RNA polymerase II subunit RPB2 146 NOT IN CONTROL IP POLR2A_228kdBand RPAP2 Isoform 1 of RNA polymerase II-associated protein 2 24 NOT IN CONTROL IP POLR2A_228kdBand POLR2G DNA-directed RNA polymerase II subunit RPB7 23 NOT IN CONTROL IP POLR2A_228kdBand POLR2H DNA-directed RNA polymerases I, II, and III subunit RPABC3 19 NOT IN CONTROL IP POLR2A_228kdBand POLR2C DNA-directed RNA polymerase II subunit RPB3 17 NOT IN CONTROL IP POLR2A_228kdBand POLR2J RPB11a protein 7 NOT IN CONTROL IP POLR2A_228kdBand POLR2E DNA-directed RNA polymerases I, II, and III subunit RPABC1 8 NOT IN CONTROL IP POLR2A_228kdBand POLR2I DNA-directed RNA polymerase II subunit RPB9 9 NOT IN CONTROL IP POLR2A_228kdBand ALMS1 ALMS1 3 NOT IN CONTROL IP POLR2A_228kdBand POLR2D DNA-directed RNA polymerase II subunit RPB4 6 NOT IN CONTROL IP POLR2A_228kdBand GRINL1A;Gcom1 Isoform 12 of Protein GRINL1A 6 NOT IN CONTROL IP POLR2A_228kdBand RECQL5 Isoform Beta of ATP-dependent DNA helicase Q5 3 NOT IN CONTROL IP POLR2A_228kdBand POLR2L DNA-directed RNA polymerases I, II, and III subunit RPABC5 5 NOT IN CONTROL IP POLR2A_228kdBand KRT6A Keratin, type II cytoskeletal 6A 3 NOT IN CONTROL IP POLR2A_228kdBand POLR2K DNA-directed RNA polymerases I, II, and III subunit RPABC4 2 NOT IN CONTROL IP POLR2A_228kdBand RFC4 Replication factor C subunit 4 1 NOT IN CONTROL IP POLR2A_228kdBand RFC2