Topical Formulations and Critical Quality Attributes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
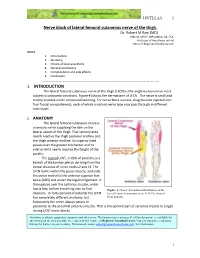
Nerve Block of Lateral Femoral Cutaneous Nerve of the Thigh
18VTLLAA 1 Nerve block of lateral femoral cutaneous nerve of the thigh. Dr. Robert M Raw (MD) . MBChB, MFGP, MPraxMed, DA, FCA. Professor of Anesthesia retired Editor of Regional-Anesthesia.Com INDEX. 1. Introduction 2. Anatomy 3. Choice of local anesthetic 4. General indications 5. Complications and side effects 6. Conclusion ------------------------------------------------------------------------------------ 1. INTRODUCTION The lateral femoral cutaneous nerve of the thigh (LFCN) is the single human nerve most subject to anatomic variations. Figure #1shows the dermatome of LFCN. The nerve is small and mostly invisible under ultrasound scanning. For nerve block success, drug must be injected into four fascial compartments, each of which a variant nerve type may pass through in different individuals. 2. ANATOMY The lateral femoral cutaneous nerve is a sensory nerve supplying the skin on the lateral aspect of the thigh. That sensory area nearly reaches the thigh posterior midline and the thigh anterior midline. Its superior limit passes over the greater trochanter and its inferior limit nearly reaches the height of the patella. The typical LCNT, in 60% of patients, is a branch of the lumbar plexus deriving from the dorsal divisions of nerve roots L2 and L3. The LFCN forms within the psoas muscle, and exits the pelvis medial to the anterior superior iliac spine (ASIS) and under the inguinal ligament. It then passes over the sartorius muscle, under fascia lata, before branching into its final Figure 1. Classic dermatomal distribution of the divisions. In forty percent of patients the LFCN lateral femoral cutaneous nerve (LFCN), derived has completely different anatomy, but from Sobotta. fortunately the nerve always passes in proximity to the proximal sartorius muscle. -

Gel-Syn™ Product Information Caution
GEL-SYN™ PRODUCT INFORMATION CAUTION: Federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner). CONTENT Each 1 mL of Gel-Syn contains: Sodium Hyaluronate: 8.4 mg Sodium Chloride: 8.5 mg Sodium Phosphate, Dibasic: 0.16 mg Sodium Phosphate, Monobasic: 0.045 mg Water for Injection: q.s. to 1.0 mL DESCRIPTION Gel-Syn is a sterile, buffered solution of highly purified sodium hyaluronate with a molecular weight of approximately 1100 kDa, obtained through fermentation of Streptococci of Lancefield groups A and C and chemically unmodified. INDICATION Gel-Syn is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy and simple analgesics (e.g., acetaminophen). CONTRAINDICATIONS • Do not administer to patients with known hypersensitivity (allergy) to sodium hyaluronate preparations. • Do not inject Gel-Syn into the knees of patients having knee joint infections or skin diseases or infections in the area of the injection site. WARNINGS • Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because sodium hyaluronate can precipitate in their presence. • Inject into the synovial space only. Do not inject by intravascular route. • Do not inject outside the synovial space or into the synovial tissue or capsule. An extra- articular injection of the product can cause local adverse events. PRECAUTIONS General • The safety and effectiveness of Gel-Syn in locations other than the knee, and for conditions other than osteoarthritis, have not been established. • Strict aseptic administration technique must be followed. -

Pricing Sheet
Trusted Service. Proven Savings. Pharmacy: (325) 704-5222 Fax: (325) 777-4819 17 Windmill Circle Suite B Abilene, TX 79606 bigcountry.clarxpharmacy.com SureScript ID: 1171544616 Dermatology Compounds UPDATED 9/3/2020 Medication QTY Price Fluorouracil/ Calcipotriene Cream 4.5/ 0.005% 30 g $55 Fluorouracil Cream 4.5% or 1.5% 30 g $50 Wart Peel Cream (Salicylic Acid/ Fluorouracil 17/ 2%) 30 g $45 Ivermectin Lotion 1.5% 30 g $45 Double Rosacea Cream (Metronidazole/ Ivermectin 1/ 1%) 30 g $55 Triple Rosacea Cream (Azelaic Acid/ Metronidazole/ Ivermectin 15/ 1/ 1%) 30 g $45 Azelaic Acid Cream 17% 30 g $45 Calcipotriene/ Clobetasol Cream 0.005/ 0.045% 60 g $85 Clioquinol/ Hydrocortisone Cream 1/ 1% 30 g $55 Tretinoin Micro Cream 0.033/ 0.055/ 0.09% 45 g $55 Clindamycin/ Tretinoin Micro Cream 1/0.03% 30 g $75 Triamcinolone/ Hydroquinone/ Tretinoin Cream 0.025/ 4/ 0.05% 30 g $55 Hydrocortisone/ Hydroquinone/ Tretinoin Cream 2.5/ 8/ 0.05% 30 g $55 Hydroquinone/ Kojic Acid Cream 12/ 6% or 10/ 3% 30 g $65 Hydroquinone/ Tretinoin Cream 4/ 0.05% 30 g $65 Squaric Acid Solution 0.1 / 0.01 / 0.001% 15 ml $85 Urea Cream 35% 90 g $45 Dapsone 6% Cream 60 g $55 Costmetic Numbing Oint. (Lidocain 23%/Tetracain 7%) 60g $35 General topical compound pricing for other compounds QTY Price Variations to the compounds above will fall into the pricing below. One Active Ingredient 30g $79 Two Active Ingredients 30g $89 Three Active Ingredients 30g $99 *This applies to most compounds (90%) not all, if exceptionally expensive we will call the office to discuss* All cash, no rebates or insurance to mess with! Medicare patients get medications at these great prices too! Free Delivery in 3-5 business days for compounds Page 1 Trusted Service. -

Physical-Chemical Characteristics of Whitening Toothpaste and Evaluation of Its Effects on Enamel Roughness
Dental materials Physical-chemical characteristics of whitening toothpaste and evaluation of its effects on enamel roughness Sérgio Paulo Hilgenberg(a) Abstract: This in vitro study evaluated the physical-chemical characteris- (a) Shelon Cristina Souza Pinto tics of whitening toothpastes and their effect on bovine enamel after ap- Paulo Vitor Farago(b) Fábio André Santos(a) plication of a bleaching agent (16% carbamide peroxide). Physical-chem- Denise Stadler Wambier(a) ical analysis was made considering mass loss by desiccation, ash content and pH of the toothpastes. Thirty bovine dental enamel fragments were prepared for roughness measurements. The samples were subjected to (a) Department of Dentistry, School of Dentistry, Ponta Grossa State University, bleaching treatments and simulated brushing: G1. Sorriso Dentes Brancos Ponta Grossa, PR, Brazil. (Conventional toothpaste), G2. Close-UP Whitening (Whitening tooth- (b) Department of Pharmacy, School of paste), and G3. Sensodyne Branqueador (Whitening toothpaste). The av- Dentistry, Ponta Grossa State University, erage roughness (Ra) was evaluated prior to the bleaching treatment and Ponta Grossa, PR, Brazil. after brushing. The results revealed differences in the physical-chemical characteristics of the toothpastes (p < 0.0001). The final Ra had higher values (p < 0.05) following the procedures. The mean of the Ra did not show significant differences, considering toothpaste groups and bleach- ing treatment. Interaction (toothpaste and bleaching treatment) showed significant difference -

An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: a Review
Muthadi Radhika Reddy /J. Pharm. Sci. & Res. Vol. 12(7), 2020, 925-940 An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review Muthadi Radhika Reddy1* 1School of pharmacy, Gurunanak Institute of Technical Campus, Hyderabad, Telangana, India and Department of Pharmacy, Gandhi Institute of Technology and Management University, Vizag, Andhra Pradesh, India INTRODUCTION 2. Useful in situations where rapid onset of action Fast dissolving drug delivery systems were first developed required such as in motion sickness, allergic attack, in the late 1970s as an alternative to conventional dosage coughing or asthma forms. These systems consist of solid dosage forms that 3. Has wide range of applications in pharmaceuticals, Rx disintegrate and dissolve quickly in the oral cavity without Prescriptions and OTC medications for treating pain, the need of water [1]. Fast dissolving drug delivery cough/cold, gastro-esophageal reflux disease,erectile systems include orally disintegrating tablets (ODTs) and dysfunction, sleep disorders, dietary supplements, etc oral thin films (OTFs). The Centre for Drug Evaluation [4] and Research (CDER) defines ODTs as,“a solid dosage 4. No water is required for the administration and hence form containing medicinal substances which disintegrates suitable during travelling rapidly, usually within a matter of seconds, when placed 5. Some drugs are absorbed from the mouth, pharynx upon the tongue” [2]. USFDA defines OTFs as, “a thin, and esophagus as the saliva passes down into the flexible, non-friable polymeric film strip containing one or stomach, enhancing bioavailability of drugs more dispersed active pharmaceutical ingredients which is 6. May offer improved bioavailability for poorly water intended to be placed on the tongue for rapid soluble drugs by offering large surface area as it disintegration or dissolution in the saliva prior to disintegrates and dissolves rapidly swallowing for delivery into the gastrointestinal tract” [3]. -

Acrylamide Polymerization — a Practical Approach
electrophoresis tech note 1156 Acrylamide Polymerization — A Practical Approach Paul Menter, Bio-Rad Laboratories, 2000 Alfred Nobel Drive, Polyacrylamide Gel Polymerization Hercules, CA 94547 USA AcrylamideBis Polyacrylamide Introduction The unparalleled resolution and flexibility possible with CH2 CH + CH2 CH CH2 CH CH2 CH CH2 CH polyacrylamide gel electrophoresis (PAGE) has led to its CO CO CO CO CO widespread use for the separation of proteins and nucleic NH2 NH NH2 NH2 NH acids. Gel porosity can be varied over a wide range to meet CH2 CH2 specific separation requirements. Electrophoresis gels and NH NH NH NH buffers can be chosen to provide separation on the basis of CO 2 2 CO CO C O charge, size, or a combination of charge and size. CH2 CH CH2 CH CH2 CH CH2 CH The key to mastering this powerful technique lies in the polymerization process itself. By understanding the important Purity of Gel-Forming Reagents parameters, and following a few simple guidelines, the novice Acrylamide can become proficient and the experienced user can optimize Gel-forming reagents include the monomers, acrylamide and bis, separations even further. as well as the initiators, usually ammonium persulfate and TEMED or, occasionally, riboflavin and TEMED. On a molar This bulletin takes a practical approach to the preparation of basis, acrylamide is by far the most abundant component in the polyacrylamide gels. Its purpose is to provide the information monomer solution. As a result, acrylamide may be the primary required to achieve reproducible, controllable polymerization. source of interfering contaminants (Dirksen and Chrambach For those users interested only in the “bare essentials,” the 1972). -

Sucralfate Enemas
Sucralfate Enemas Sucralfate enemas are used to treat inflammation of the rectum (the lower part of the large intestine leading to the anus); most commonly this is for radiation proctitis; bowel inflammation following radiotherapy. This treatment can help treat bleeding due to the inflammation, and can be continued for up to 24 weeks; you can stop when the bleeding stops. This fact sheet explains how you can administer the enema yourself, using a syringe and catheter. The hospital or community pharmacy can provide the Sucralfate enema, and you will need to ask your GP or practise nurse to supply the equipment you will need. Your practise nurse can help you practice preparing the enema, and could help you with administering it at home, if you find this difficult. 1. Using the syringe provided, draw up 10mls of the Sucralfate Suspension (2g) and then draw up 10mls of warm water and mix. Ensure that any excess air is expelled from the syringe. 2. Attach the catheter provided to the syringe tip and lubricate the end of the catheter with lubricating gel provided. 3. Lie on your left side with both knees bent. This position will help the flow of liquid in to the rectum and aid enema retention. 4. Position a towel underneath yourself to catch any leakage of fluid once the enema has been administered. 5. With a steady pressure, gently insert the catheter to a depth of approximately 10cm into the rectum. 6. Press down on the barrel of the syringe slowly, using a steady and even pressure, until the entire contents of the syringe have been administered. -

Cream LOTRISONE® Lotion (Clotrimazole and Betamethasone Dipropionate)
LOTRISONE® Cream LOTRISONE® Lotion (clotrimazole and betamethasone dipropionate) FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. NOT RECOMMENDED FOR PATIENTS UNDER THE AGE OF 17 YEARS AND NOT RECOMMENDED FOR DIAPER DERMATITIS. DESCRIPTION LOTRISONE® Cream and Lotion contain combinations of clotrimazole, a synthetic antifungal agent, and betamethasone dipropionate, a synthetic corticosteroid, for dermatologic use. Chemically, clotrimazole is 1–(o-chloro-α,α-diphenylbenzyl) imidazole, with the empirical formula C22H17CIN2, a molecular weight of 344.84, and the following structural formula: Clotrimazole is an odorless, white crystalline powder, insoluble in water and soluble in ethanol. Betamethasone dipropionate has the chemical name 9-fluoro-11β,17,21- trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate, with the empirical formula C28H37FO7, a molecular weight of 504.59, and the following structural formula: 1 LRN# 000370-LOS-MTL-USPI-1 Betamethasone dipropionate is a white to creamy white, odorless crystalline powder, insoluble in water. Each gram of LOTRISONE Cream contains 10 mg clotrimazole and 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone), in a hydrophilic cream consisting of purified water, mineral oil, white petrolatum, cetyl alcohol plus stearyl alcohol, ceteareth-30, propylene glycol, sodium phosphate monobasic monohydrate, and phosphoric acid; benzyl alcohol as preservative. LOTRISONE Cream may contain sodium hydroxide. LOTRISONE Cream is smooth, uniform, and white to off-white in color. Each gram of LOTRISONE Lotion contains 10 mg clotrimazole and 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone), in a hydrophilic base of purified water, mineral oil, white petrolatum, cetyl alcohol plus stearyl alcohol, ceteareth-30, propylene glycol, sodium phosphate monobasic monohydrate, and phosphoric acid; benzyl alcohol as a preservative. -

Preparatory: 1 Venous Access and Medication Administration: 4
Preparatory: 1 Venous Access and Medication Administration: 4 W4444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444 UNIT TERMINAL OBJECTIVE 1-4 At the completion of this unit, the EMT-Critical Care Technician student will be able to safely and precisely access the venous circulation and administer medications. COGNITIVE OBJECTIVES At the completion of this unit, the EMT-Critical Care Technician student will be able to: 1-4.1 Review the specific anatomy and physiology pertinent to medication administration. (C-1) 1-4.2 Review mathematical principles. (C-1) 1-4.3 Review mathematical equivalents. (C-1) 1-4.4 Differentiate temperature readings between the Centigrade and Fahrenheit scales. (C-3) 1-4.5 Discuss formulas as a basis for performing drug calculations. (C-1) 1-4.6 Calculate oral and parenteral drug dosages for all emergency medications administered to adults, infants and children. (C-2) 1-4.7 Calculate intravenous infusion rates for adults, infants, and children. (C-2) 1-4.8 Discuss legal aspects affecting medication administration. (C-1) 1-4.9 Discuss the "six rights" of drug administration and correlate these with the principles of medication administration. (C-1) 1-4.10 Discuss medical asepsis and the differences between clean and sterile techniques. (C-1) 1-4.11 Describe use of antiseptics and disinfectants. (C-1) 1-4.12 Describe the use of universal precautions and body substance isolation (BSI) procedures when administering a medication. (C-1) 1-4.13 Describe the indications, equipment needed, techniques utilized, precautions, and general principles of peripheral venous cannulation (Including saline locks). (C-1) 1-4.14 Describe the indications, equipment needed, techniques utilized, precautions, and general principles of intraosseous needle placement and infusion. -

Handbook ESRA
TECHNIQUES HEAD & NECK 4 Intracranial surgery p. 3 Eye blocks p. 5 Face anatomy p. 16 Face particularity p. 23 Ophtalmic nerve blocks p. 27 Maxillary nerve blocks p. 33 Mandibular nerve blocks p. 46 THORAX & ABDOMEN 50 Epidural anaesthesia in Cardio-thoracic surgery p. 50 Ilioinguinal-Iliohypogastric block p. 55 Peri-umbilical & Rectus sheath block p. 57 Pudendal block p. 58 UPPER LIMB 61 Choice of a technique p. 61 Brachial plexus anatomy p. 65 Interscalen block p. 68 Supraclavicular blocks p. 73 Infraclavicular blocks p. 80 Axillary block p. 83 LOWER LIMB 90 Lumbar plexus block p. 90 Iliofascial block p. 100 Obturator block p. 102 Sciatic blocks o Sciatic blocks - parasacral nerve approach p. 109 o Sciatic blocks - posterior popliteal approach p. 115 Ankle blocks p. 119 AXIAL BLOCKS 123 Lumbar epidural p. 123 OBSTETRICS AXIAL BLOCKS 126 Epidural p. 126 PERIPHERAL BLOCKS Pudendal block p. 58 2 Aknowledgement The provenience of the materials included in this handbook is from the Learning Zone on the official site of “European Society of Regional Anesthesia and Pain Therapy”. http://www.esra-learning.com/ 2007 3 HEAD & TABLE OF CONTENTS NECK • Intracranial surgery • Eye blocks • Face anatomy • Face particularity • Ophtalmic nerve blocks • Maxillary nerve blocks • Mandibular nerve blocks • Cervical plexus blocks HEAD & INTRACRANIAL SURGERY NECK Paul J. Zetlaoui, M.D. Kremlin-Bicetre - France In intra-cranial neurosurgery, scalp infiltration aims to prevent systematic and cerebral hemodynamic variations, contemporary of skin incision. The potential morbidity of these hypertension-tachycardia episodes, even in patients profoundly anaesthetized, is secondary in the increase of the cerebral blood flow and in its deleterious consequences on intra-cranial pressure in these compromised patients. -

The Development of an Intramuscular Injection Simulation for Nursing Students
Open Access Technical Report DOI: 10.7759/cureus.12366 The Development of an Intramuscular Injection Simulation for Nursing Students Julia Micallef 1 , Artur Arutiunian 1 , Adam Dubrowski 1 1. Health Sciences, Ontario Tech University, Oshawa, CAN Corresponding author: Adam Dubrowski, [email protected] Abstract Intramuscular (IM) injections are preferred over subcutaneous injections for administering medicine such as epinephrine and vaccines as the muscle tissue contains an increased vascular supply that provides ideal absorption of the drug being administered. However, administering an IM injection requires clinical judgment when choosing the injection site, understanding the relevant anatomy and physiology as well as the principles and techniques for administering an IM injection. Therefore, it is essential to learn and perform IM injections using injection simulators to practice the skill before administering to a real patient. Current IM injection simulators either favor realism at the expense of standardization or are expensive but do not provide a realistic experience. Therefore, it is imperative to develop an inexpensive but realistic intramuscular injection simulator that can be used to train nursing students so that they can be prepared for when they enter the clinical setting. This technical report aims to provide an overview of the development of an inexpensive and realistic deltoid simulator geared to teach nursing students the skill of IM injections. After development, the IM simulators were tested and validated by practicing nurses. An 18-item survey was administered to the nurses, and results indicated positive feedback about the realism of the simulator, in comparison to previous models used, such as the Wallcur® PRACTI-Injecta Pads (Wallcur LLC, San Diego, CA). -

Salicylic Acid
Treatment Guide to Common Skin Conditions Prepared by Loren Regier, BSP, BA, Sharon Downey -www.RxFiles.ca Revised: Jan 2004 Dermatitis, Atopic Dry Skin Psoriasis Step 1 - General Treatment Measures Step 1 - General Treatment Measures Step 1 • Avoid contact with irritants or trigger factors • Use cool air humidifiers • Non-pharmacologic measures (general health issues) • Avoid wool or nylon clothing. • Lower house temperature (minimize perspiration) • Moisturizers (will not clear skin, but will ↓ itching) • Wash clothing in soap vs detergent; double rinse/vinegar • Limit use of soap to axillae, feet, and groin • Avoid frequent or prolonged bathing; twice weekly • Topical Steroids Step 2 recommended but daily bathing permitted with • Coal Tar • Colloidal oatmeal bath products adequate skin hydration therapy (apply moisturizer • Anthralin • Lanolin-free water miscible bath oil immediately afterwards) • Vitamin D3 • Intensive skin hydration therapy • Limit use of soap to axillae, feet, and groin • Topical Retinoid Therapy • “Soapless” cleansers for sensitive skin • Apply lubricating emollients such as petrolatum to • Sunshine Step 3 damp skin (e.g. after bathing) • Oral antihistamines (1st generation)for sedation & relief of • Salicylic acid itching give at bedtime +/- a daytime regimen as required Step 2 • Bath additives (tar solns, oils, oatmeal, Epsom salts) • Topical hydrocortisone (0.5%) for inflammation • Colloidal oatmeal bath products Step 2 apply od-tid; ointments more effective than creams • Water miscible bath oil • Phototherapy (UVB) may use cream during day & ointment at night • Humectants: urea, lactic acid, phospholipid • Photochemotherapy (Psoralen + UVA) Step 4 Step 3 • Combination Therapies (from Step 1 & 2 treatments) • Prescription topical corticosteroids: use lowest potency • Oral antihistamines for sedation & relief of itching steroid that is effective and wean to twice weekly.